GENOMIC
Mapping
13qA5. View the map and BAC contig (data from UCSC genome browser).
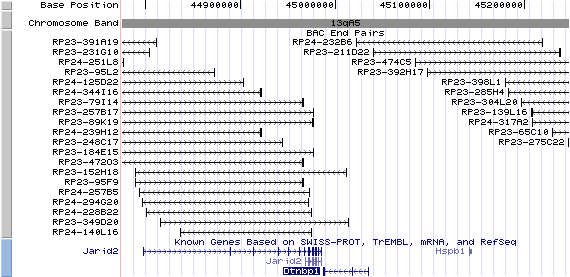
Structure
(assembly 02/2003)
Isoform a (NM_025772): 10 exons, 79,806 bp, chr13:44,409,050-44,488,855.
Isoform b (AY265461): 6 exons, 60,710 bp, chr13:44,409,055-44,469,764.
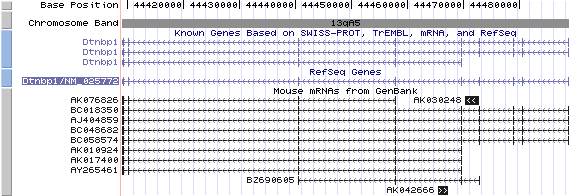
By 5'RACE, Li, et al identified that a transcript variant (AY265461) contains an alternative spliced exon 5 of NM_025772, which serves as exon 1 of transcript variant (2). The resulting isoform (b) lacks the N-terminal 81 amino acid residues of isoform (a), which is similar to human homolog dysbindin-3 (isoform c).
The figure shows the comparison of these isoforms (data from UCSC genome browser).
Regulatory Element
Search the 5'UTR and 1kb upstream regions of isoform a (seq1=human HPS7, seq2=mouse Hps7) by CONREAL with 80% Position Weight Matrices (PWMs) threshold (view results here).
TRANSCRIPT
RefSeq/ORF
a) Transcript variant 1 (NM_025772), 1,326 bp, view ORF and the alignment to genomic. Note that there is no matched genomic sequence of exon 8 because of a sequence gap.
b) Transcript variant 2 (AY265461), 1082 bp, view exon boundaries and the alignment to genomic. Note that there is no matched genomic sequence of exon 4 because of a sequence gap.
Expression Pattern
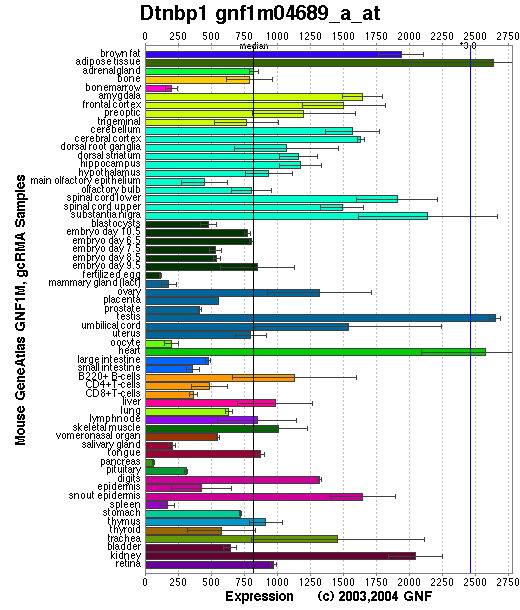
Tissue specificity: ubiquitous. The highest expression is observed in testis, liver, kidney, brain, heart and lung. Expressed at lower levels in stomach, small intestine and skeletal muscle, where it is detected at the sarcolemma. (view the GNF Gene Expression Atlas (GEA) U74A 113190_at below)
In the mouse brain, dysbindin is found primarily in axon bundles and especially in certain axon terminals, notably mossy fiber synaptic terminals in the cerebellum and hippocampus (Benson, et al (2001)). Bray, et al found that the allelic expression differences exceeded 50% in the brain, which suggests that cis-acting variation in gene expression occurs. In the mouse cerebellum, dysbindin immunoreactivity is expressed at high levels in a subset of mossy fiber synaptic glomeruli in the granular layer. Lower levels of dysbindin immunoreactivity are also detected in Purkinje cell dendrites. In the cerebellar vermis, dysbindin-immunoreactive glomeruli are restricted to an array of parasagittal stripes that bears a consistent relationship to Purkinje cell parasagittal band boundaries as defined by the expression of the respiratory isoenzyme zebrin II/aldolase c (Sillitoe, et al).
Affymetrix microarray expression pattern in SymAtlas from GNF is shown below.
PROTEIN
Sequence
Dysbindin isoform a (NP_080048): 352aa, ExPaSy NiceProt view of Swiss-Prot: Q91WZ8.
Dysbindin isoform b ( AAP91871): 271aa.
Synonyms: Dystrobrevin binding protein 1; Hermansky-Pudlak syndrome 7 protein homolog.
Ortholog
| Species | Human | Zebrafish | Drosophila |
| GeneView | HPS7/DTNBP1 | 394109 | CG6856 |
| Protein | NP_115498(351aa) | NP_957428 (362aa) | Q9VVT5 (288aa) |
| Identities | 74%/343aa | 57%/345aa | 25%/212aa |
View multiple sequence alignment (PDF file) by ClustalW and GeneDoc.
Domain
(1)Dysbindin domain: 173-325, CDD: 23538 (Pfam04440).
(2) Domains predicted by SMART(isoform a):
a) coiled coil: 92-175
b) low complexity: 286-301
c) low complexity: 340-351
(3) Transmembrane domains predicted by SOSHI(isoform a): none (predicted as a soluble protein).
(4) Graphic view of InterPro domain structure.
Motif/Site
(1) Predicted results by ScanProsite(isoform a):
a) Protein kinase C phosphorylation site [pattern] [Warning: pattern with a high probability of occurrence]:
4 - 6 TlR, 24 - 26 SdK, 157 - 159 SyK, 161 - 163 SkR, 178 - 180 TqK, 331 - 333 TdR
b) N-myristoylation site [pattern] [Warning: pattern with a high probability of occurrence]:
226 - 231 GSmsSM, 259 - 264 GGedNI
c) Tyrosine sulfation site [rule] [Warning: rule with a high probability of occurrence]:
48 - 62 glellsrYedawaal
d) cAMP- and cGMP-dependent protein kinase phosphorylation site [pattern] [Warning: pattern with a high probability of occurrence]:
89 - 92 KKrT, 90 - 93 KRtS, 333 - 336 RKsT
e) Leucine zipper pattern [pattern] [Warning: pattern with a high probability of occurrence]:
97 - 118 LqgqlqqLpallqdLeslmasL
(2) Predicted results of subprograms by PSORT II(isoform a):
a) N-terminal signal peptide: none
b) KDEL ER retention motif in the C-terminus: none
c) ER Membrane Retention Signals: none
d) VAC possible vacuolar targeting motif: none
e) Actinin-type actin-binding motif: type 1: none; type 2: none
f) Prenylation motif: none
g) memYQRL transport motif from cell surface to Golgi: none
h) Tyrosines in the tail: none
i) Dileucine motif in the tail: none
3D Model
(1) ModBase: none.
(2) 3D models of isoform (a) predicted by SPARKS (fold recognition) below. View the models by PDB2MGIF.
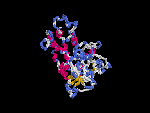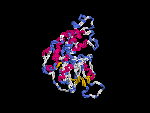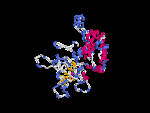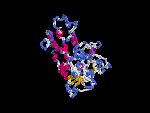
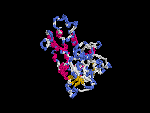
2D-PAGE
This protein does not exist in the current release of SWISS-2DPAGE.
Computed theoretical MW=39,651Da, pI=4.59 (isoform a).
Computed theoretical MW=30,581Da, pI=4.35 (isoform b).
When probed with anti-dysbindin antibody on Western blots, a ~51 kD band presents in all the tissues examined, while a ~38 kD band is apparent in brain which is also missing in sdy/sdy brain (Benson, et al; Li, et al).
FUNCTION
Ontology
a) Biological process: visual perception, muscle development.
b) Plays a role in the biogenesis of lysosome-related organelles such as platelet dense granule and melanosomes (view diagram of BLOC-1 pathway here).
Location
Mostly cytoplasmic. Interaction with the DPC complex suggests that dysbindin may associated with plasma membrane (Benson, et al (2001)). Dysbindin-1 is likely a nucleocytoplasmic shuttling protein and translocated to the nucleus upon treatment with leptomycin B, an inhibitor of exportin-1/CRM1-mediated nuclear export. Dysbindin-1 harbors a functional nuclear export signal necessary for its nuclear export, and the nucleocytoplasmic shuttling of dysbindin-1 affects its regulation of synapsin I expression (Fei, et al).
Interaction
Binds to alpha and beta dystrobrevins that are components of the dystrophin-associated protein complex (DPC) (Benson, et al(2001); Li, et al (2003)) (view diagram of DPC and BLOC-1 interaction here). Nazarian, et al demonstrated direct interaction involving coiled-coil-forming regions in both dysbindin and the dystrobrevins. However, recombinant proteins bearing the coiled-coil-forming regions of the dystrobrevins failed to bind endogenous BLOC-1 from HeLa cells or mouse brain or muscle, suggesting that dysbindin assembled into BLOC-1 is not a physiological binding partner of the dystrobrevins. Benson, et al (2004) showed that dysbindin binds to a novel 413-kDa protein, myospryn, which is expressed in cardiac and skeletal muscle. The C terminus of myospryn contains BBC, FN3, and SPRY domains in a configuration reminiscent of the tripartite motif protein family, as well as the dysbindin-binding site and a region mediating self-association. Desmin binds to myospryn,dysbindin and pallidin. The N-terminal 1-103 fragment of desmin binds to myospryn carboxyl terminus through the SPRY domain (Kouloumenta, et al ). Yeast two-hybrid screening identified the direct interaction of a novel RING finger protein RNF151 and dysbindin, which was confirmed by the co-immunoprecipitation of the proteins and by their co-localization in intact cells, suggesting a role in acrosome formation (Nian, et al ). Yin, et al suggested that the acidic domain of dysbindin and its paralogs in humans may function to recruit casein kinase-1 isoforms to protein complexes involved in multiple biological functions. In the yeast two-hybrid system, TRIM32 binds and ubiquitinates dysbindin (Locke, et al ). A specific interaction between the domains of DISC1 (316-597) and dysbindin (82-173) (Ottis, et al ).
Dysbindin interacts with pallidin, muted and snapin (Li, et al (2003); Starcevic, et al). Dysbindin is a subunit of the biogenesis of lysosome-related organelles complex 1 (BLOC-1), which is composed of the products of Dtnbp1 and seven other HPS genes, mu, pa, cno, rp, Snapap, Blos1, Blos2 (Ciciotte, et al; Falcon-Perez , et al; Li, et al (2003); Moriyama, et al; Starcevic, et al) (view diagram of BLOC-1 complex here). Within BLOC-1, the same 69-residue region of dysbindin that is sufficient for dystrobrevin binding in vitro also contains the binding sites for pallidin and snapin, and at least part of the muted-binding interface (Nazarian, et al). Tissue fractionation of whole mouse brains and human hippocampal formations revealed that both dysbindin-1 and snapin are concentrated in tissue enriched in synaptic vesicle membranes and less commonly in postsynaptic densities. It is not detected in presynaptic tissue fractions lacking synaptic vesicles (Talbot, et al (2006) ).
BLOC-1 is thought to be involved in endosomal transport. Comparative proteomics of clathrin-coated vesicles (CCVs) have identified seven components of BLOC-1 (Borner, et al). BLOC-1 and AP-3 protein complexes affect the targeting of SNARE and non-SNARE AP-3 cargoes and suggest a function of the BLOC-1 complex in membrane protein sorting (Salazar, et al). BLOC-1 interacts physically and functionally with AP-3 to facilitate the trafficking of a known AP-3 cargo, CD63, and of tyrosinase-related protein 1 (Tyrp1). BLOC-1 also interacts with BLOC-2 to facilitate Tyrp1 trafficking by a mechanism apparently independent of AP-3 function. Both BLOC-1 and -2 localize mainly to early endosome-associated tubules as determined by immunoelectron microscopy (Di Pietro, et al). BLOC-1-deficient melanocytes accumulate the melanosomal protein Tyrp1, but not other melanosomal proteins, in endosomal vacuoles and the cell surface due to failed biosynthetic transit from early endosomes to melanosomes and consequent increased endocytic flux. Melanocytes from HPS model mice lacking a different protein complex, BLOC-2, accumulate Tyrp1 in distinct downstream endosomal intermediates, suggesting that BLOC-1 and BLOC-2 act sequentially in the same pathway. By contrast, intracellular Tyrp1 is correctly targeted to melanosomes in melanocytes lacking another HPS-associated protein complex, AP-3 (Setty, et al). Dysbindin formed a complex with the AP-3 complex through the direct binding to its mu subunit (Taneichi-Kuroda, et al).
Overexpression of dysbindin induced the expression of two pre-synaptic proteins, SNAP25 and synapsin I, and increased extracellular basal glutamate levels and release of glutamate evoked by high potassium. Conversely, knockdown of endogenous dysbindin protein by small interfering RNA (siRNA) resulted in the reduction of pre-synaptic protein expression and glutamate release, suggesting that dysbindin might influence exocytotic glutamate release via upregulation of the molecules in pre-synaptic machinery (Numakawa, et al). Munc18-1 was co-immunoprecipitated with dysbindin from rat brain lysate, and directly interacted with dysbindin in vitro. In primary cultured rat hippocampal neurons, a part of dysbindin was co-localized with Munc18-1 at pre-synaptic terminals (Hikita, et al). In situ hybridization analysis showed the mRNA expression of dysbindin in the mouse substantia nigra. Suppression of dysbindin expression in PC12 cells resulted in an increase of the expression of SNAP25, and increased the release of dopamine. On the other hand, up-regulation of dysbindin expression in PC12 cells showed a tendency to decrease the expression of SNAP25. These suggest that dysbindin might regulate the dopamine release in dopaminergic cells via modulation of the expression of SNAP25 (Kumamoto, et al).
DTNBP1 siRNA increased cell surface DRD2 and blocked dopamine-induced DRD2 internalization. MUTED siRNA produced similar effects. In contrast, decreased dysbindin did not change dopamine D1 receptor (DRD1) levels, or its basal or dopamine-induced internalization (Iizuka, et al). Dysbindin co-immunoprecipitated with GASP-1 (or GPRASP-1), a cytoplasmic protein shown previously to modulate lysosomal trafficking of D2 dopamine and delta opioid receptors by direct interaction (Marley, et al).
GST pull-down from human neuroblastoma lysates showed an association of dysbindin-1 with the DNA-dependent protein kinase (DNA-PK) complex. The DNA-PK complex interacts only with splice isoforms A and B, but not with C which lacks exons 1-6. Isoforms A and B localized in nucleus, where the kinase complex exist, whereas the isoform C was found exclusively in cytosol. Phosphorylation assay shows that the DNA-PK complex phosphorylated dysbindin-1 isoforms A and B, suggesting that DNA-PK regulates the dysbindin-1 isoforms A and B by phosphorylation in nucleus (Oyama, et al).
In the pathogeneisis of dysbindin-related schizophrenia, neurodevelopmenal defects have been noted. dysbindin-1, WAVE2 and Abi-1 form a ternary complex, and dysbindin-1 promotes the binding of WAVE2 to Abi-1. It is suggested that dysbindin-1 at the postsynapse regulates dendritic spine morphogenesis through the interaction with WAVE2 and Abi-1 (Ito, et al). In addition, necdin is a binding partner of dysbindin-1 by using a yeast two-hybrid screen. Dysbindin-1 recruits necdin to the cytoplasm, thereby attenuating the repressive effects of necdin on p53 transcriptional activity. Knockdown of dysbindin-1, like knockdown of p53, greatly decreases the expressions of the p53 target genes coronin 1b and rab13, which are required for neurite outgrowth (Ma, et al).
Dysbindin and DISC1 share common PPIs suggesting they may affect common biological processes and that the function of schizophrenia risk genes may converge (Camargo, et al). A direct interaction of soluble and insoluble DISC1 protein with dysbindin protein demonstrates convergence of so far considered independent mental disease genes by direct molecular interaction (Ottis, et al). Guo, et al constructed a DTNBP1 interactome. The link between dysbindin and DISC1 is suggested by the interactions with members of the exocyst, dynactin and chaperonin containing T-complex protein complexes (Mead, et al).
Besides BLOC-1 and dystrophin-associated protein complex, several molecules in the DTNBP1 network likely provide insight into the role of DTNBP1 in biological systems: retinoic acid, beta-estradiol, calmodulin and tumour necrosis factor.
HPS7p drosophila homolog CG6856 interaction information in CuraGen interaction database.
Pathway
Nguyen, et al found that the greatest percentages of immature melanosomes were observed in the BLOC-1 mutants pa and cno, which suggests the maturation of melanosome in sdy is also likely blocked between the multivesicular body stage and stage I (view diagram of melanosome blockage here). Melanosome maturation requires at least two cargo transport pathways directly from early endosomes to melanosomes, one mediated by AP-3 and another by BLOC-1 and BLOC-2 (Setty, et al).
MUTATION
Allele or SNP
1 phenotypic allele is described in MGI:2137586.
SNPs deposited in dbSNP.
Distribution
| Location | Genomic | cDNA | Protein | Type | Strain | Reference |
| Intron 5 | (intron 5 +3,702)~(intron 7 +12,376) del 38,129bp | 356-511del 156bp (del Exons 6+7) | A119-A171 del 156bp (del 52aa) | in-frame | sdy (DBA/2J) | Li, et al |
Effect
Li, et al found that although the mutant transcripts were detected in multiple tissues, the in-frame mutation causes the loss of expression of dysbindin. The loss of dysbindin destabilizes other BLOC-1 members, such as pallidin, muted, cno. Likewise, the predicted mutant protein is expected to cause the deletion of most of the coiled-coil region. The mutant protein is over-expressed in transfected cells, where it binds to pallidin and muted weakly, but not beta-dystrobrevin.
PHENOTYPE
Mutation in the Dtnbp1 gene is the cause of sandy mutant (Li, et al; Swank, et al), a mouse model of Hermansky-Pudlak syndrome type 7 (HPS-7, OMIM 607145). The sdy allele arose from DBA/2J. The strain is described in more detail in JAX Mice database (DBA/2J-Dtnbp1sdy/J). In brief, mutations at this locus result in pigmentation anomalies of the coat and eye as well as prolonged bleeding times due to platelet abnormalities. Retinal pigment epithelium (RPE) melanosomes of the BLOC-1 mutants Hps7/sdy, mu, cno are severely reduced in number. Also, unusual melanosomes, perhaps in the process of degradation, are present. Choroidal melanosomes of all three mutants are smaller, are often incompletely melanized and have irregular edges. Preliminary behavioral tests of the sdy mice were negative (Li, et al). In sdy brains, the ratios of DA to HVA, which is an index of DA turnover, were higher in the cortex and the hippocampus, but not in the hypothalamus, suggesting a possible useful candidate animal for studying the pathogenic mechanism of schizophrenia (Murotani, et al).
As DTNBP1 is a hallmark in the susceptibility gene of schizophrenia, the null mutation of Dtnbp1 in sandy mice is informative to explore its role in the development of schizophrenic symptoms in sdy mice. Chen, et al first described the abnormal synaptic ultrastructures and the impaired glutamatergic transmission in the hippocampal formation, which may lead to the schizophrenia-like symptoms in sdy mice (Feng, et al (2008)). The abnormal behaviors mimicking schizophrenia in sdy mice were reproduced in several other groups (Hattori, et al (2008); Takao, et al (2008); Bhardwaj, et al (2009); Cox, et al (2009)), supporting that the sdy mutant is an appropriate model of schizophrenia.
To study the contribution of dysbindin to schizophrenia, defects in synaptic transmission, postsynaptic modulation and neurodevelopment have been noted in the sdy mice. Several neurotransmitters, such as glutamate, dopamine, GABA, serotonin, in the cerebral cortex, midbrain, hippocampus in sdy mice have been shown abnormal release patterns (Chen, et al; Ji, et al; Kumamoto, et al; Murotani, et al; Nagai, et al). Postsynaptic receptors such as D2R, NR2A are increased in sdy brain (Iizuka, et al; Ji, et al; Tang, et al). In contrast to the role of dysbindin-1 in glutamatergic transmission, gamma-band abnormalities in schizophrenia are most often attributed to disrupted inhibition and reductions in parvalbumin-positive interneuron (PV cell) activity. PV cell immunoreactivity was reduced in sdy mice(Carlson, et al). In addition, dysbindin regulates dendritic spine morphogenesis (Ito, et al), neurite outgrowth (Ghiani, et al; Ma, et al), neuronal progenitor cell differentiation in the adult hippocampal neurogenesis (Nihonmatsu-Kikuchi, et al). Serotonin and dopamine, two major neuromodulators implicated in the pathophysiology of schizophrenia, can potentiate mossy fiber synaptic transmission probably via an increase in cAMP levels (Kobayashi, et al).
Interestingly, dysbindin-1 is up-regulated in the dystrophin-deficient muscle of a mouse model of Duchenne muscular dystrophy, the mdx mutant (Benson, et al (2001)). Moreover, Sillitoe, et al reported that there is a dramatic increase of the number of dysbindin-immunoreactive glomeruli in the posterior cerebellar vermis in the mdx mutant. Abnormal synaptic organization in the cerebellum may contribute to the neurological problems associated with muscular dystrophy and schizophrenia.
REFERENCE
- Benson MA, Newey SE, Martin-Rendon E, Hawkes R, Blake DJ. Dysbindin, a novel coiled-coil-containing protein that interacts with the dystrobrevins in muscle and brain. J Biol Chem 2001; 276: 24232-41. PMID: 11316798
- Benson MA, Tinsley CL, Blake DJ. Myospryn is a novel binding partner for dysbindin in muscle. J Biol Chem 2004; 279: 10450-8. PMID: 14688250
- Bhardwaj SK, Baharnoori M, Sharif-Askari B, Kamath A, Williams S, Srivastava LK. Behavioral characterization of dysbindin-1 deficient sandy mice. Behav Brain Res 2009; 197: 435-41. PMID: 18984010
- Borner GH, Harbour M, Hester S, Lilley KS, Robinson MS. Comparative proteomics of clathrin-coated vesicles. J Cell Biol 2006; 175: 571-8. PMID: 17116749
- Bray NJ, Buckland PR, Owen MJ, O'Donovan MC. Cis-acting variation in the expression of a high proportion of genes in human brain. Hum Genet 2003; 113: 149-53. PMID: 12728311
- Carlson GC, Talbot K, Halene TB, Gandal MJ, Kazi HA, Schlosser L, Phung QH, Gur RE, Arnold SE, Siegel SJ. Dysbindin-1 mutant mice implicate reduced fast-phasic inhibition as a final common disease mechanism in schizophrenia. Proc Natl Acad Sci U S A 2011; 108: E962-70. PMID: 21969553
- Camargo LM, Collura V, Rain JC, Mizuguchi K, Hermjakob H, Kerrien S, Bonnert TP, Whiting PJ, Brandon NJ. Disrupted in Schizophrenia 1 Interactome: evidence for the close connectivity of risk genes and a potential synaptic basis for schizophrenia. Mol Psychiatry 2007; 12: 74-86. PMID: 17043677
- Chen XW, Feng YQ, Hao CJ, Guo XL, He X, Zhou ZY, Guo N, Huang HP, Xiong W, Zheng H, Zuo PL, Zhang CX, Li W, Zhou Z. DTNBP1, a schizophrenia susceptibility gene, affects kinetics of transmitter release. J Cell Biol 2008; 181: 791-801. PMID: 18504299
- Ciciotte SL, Gwynn B, Moriyama K, Huizing M, Gahl WA, Bonifacino JS, Peters LL. Cappuccino, a mouse model of Hermansky-Pudlak syndrome, encodes a novel protein that is part of the pallidin-muted complex (BLOC-1). Blood 2003; 101: 4402-7. PMID: 12576321
- Cox MM, Tucker AM, Tang J, Talbot K, Richer DC, Yeh L, Arnold SE. Neurobehavioral abnormalities in the dysbindin-1 mutant, sandy, on a C57BL/6J genetic background. Genes Brain Behav 2009; 8: 390-7.PMID: 19220483
- Di Pietro SM, Falcon-Perez JM, Tenza D, Setty SR, Marks MS, Raposo G, Dell'Angelica EC. BLOC-1 interacts with BLOC-2 and the AP-3 complex to facilitate protein trafficking on endosomes. Mol Biol Cell 2006; 17: 4027-38. PMID: 16837549
- Falcon-Perez JM, Starcevic M, Gautam R, Dell'Angelica EC. BLOC-1, a novel complex containing the pallidin and muted proteins involved in the biogenesis of melanosomes and platelet-dense granules. J Biol Chem 2002; 277: 28191-9. PMID: 12019270
- Fei E, Ma X, Zhu C, Xue T, Yan J, Xu Y, Zhou J, Wang G. Nucleocytoplasmic shuttling of dysbindin-1, a schizophrenia-related protein, regulates synapsin I expression. J Biol Chem 2010; 285: 38630-40. PMID: 20921223
- Feng YQ, Zhou ZY, He X, Wang H, Guo XL, Hao CJ, Guo Y, Zhen XC, Li W. Dysbindin deficiency in sandy mice causes reduction of snapin and displays behaviors related to schizophrenia. Schizophr Res 2008; 106: 218-28. PMID: 18774265
- Ghiani CA, Starcevic M, Rodriguez-Fernandez IA, Nazarian R, Cheli VT, Chan LN, Malvar JS, de Vellis J, Sabatti C, Dell'Angelica EC. The dysbindin-containing complex (BLOC-1) in brain: developmental regulation, interaction with SNARE proteins and role in neurite outgrowth. Mol Psychiatry 2010; 15: 204-15. PMID: 19546860
- Guo AY, Sun J, Riley BP, Thiselton DL, Kendler KS, Zhao Z. The dystrobrevin-binding protein 1 gene: features and networks. Mol Psychiatry 2009; 14: 18-29. PMID: 18663367
- Hattori S, Murotani T, Matsuzaki S, Ishizuka T, Kumamoto N, Takeda M, Tohyama M, Yamatodani A, Kunugi H, Hashimoto R. Behavioral abnormalities and dopamine reductions in sdy mutant mice with a deletion in Dtnbp1, a susceptibility gene for schizophrenia. Biochem Biophys Res Commun 2008; 373: 298-302. PMID: 18555792
- Hikita T, Taya S, Fujino Y, Taneichi-Kuroda S, Ohta K, Tsuboi D, Shinoda T, Kuroda K, Funahashi Y, Uraguchi-Asaki J, Hashimoto R, Kaibuchi K. Proteomic analysis reveals novel binding partners of dysbindin, a schizophrenia-related protein. J Neurochem 2009; 110: 1567-74. PMID: 19573021
- Iizuka Y, Sei Y, Weinberger DR, Straub RE. Evidence that the BLOC-1 protein dysbindin modulates dopamine D2 receptor internalization and signaling but not D1 internalization. J Neurosci 2007; 27: 12390-5. PMID: 17989303
- Ito H, Morishita R, Shinoda T, Iwamoto I, Sudo K, Okamoto K, Nagata K. Dysbindin-1, WAVE2 and Abi-1 form a complex that regulates dendritic spine formation. Mol Psychiatry 2010; 15: 976-86. PMID: 20531346
- Ji Y, Yang F, Papaleo F, Wang HX, Gao WJ, Weinberger DR, Lu B. Role of dysbindin in dopamine receptor trafficking and cortical GABA function. Proc Natl Acad Sci U S A. 2009; 106: 19593-8. PMID: 19887632
- Kobayashi K, Umeda-Yano S, Yamamori H, Takeda M, Suzuki H, Hashimoto R. Correlated alterations in serotonergic and dopaminergic modulations at the hippocampal mossy fiber synapse in mice lacking dysbindin. PLoS One 2011; 6: e18113. PMID: 21448290
- Kouloumenta A, Mavroidis M, Capetanaki Y. Proper perinuclear localization of the TRIM-like protein myospryn requires its binding partner desmin. J Biol Chem 2007; 282: 35211-21.PMID: 17872945
- Kumamoto N, Matsuzaki S, Inoue K, Hattori T, Shimizu S, Hashimoto R, Yamatodani A, Katayama T, Tohyama M. Hyperactivation of midbrain dopaminergic system in schizophrenia could be attributed to the down-regulation of dysbindin. Biochem Biophys Res Commun 2006; 345: 904-9. PMID: 16701550
- Li W, Zhang Q, Oiso N, Novak EK, Gautam R, O'Brien EP, Tinsley CL, Blake DJ, Spritz RA, Copeland NG, Jenkins NA, Amato D, Roe BA, Starcevic M, Dell'Angelica EC, Elliott RW, Mishra V, Kingsmore SF, Paylor RE, Swank RT. Hermansky-Pudlak syndrome type 7 (HPS-7) results from mutant dysbindin, a member of the biogenesis of lysosome-related organelles complex 1 (BLOC-1). Nat Genet 2003; 35: 84-9. PMID: 12923531
- Locke M, Tinsley CL, Benson MA, Blake DJ. TRIM32 is an E3 ubiquitin ligase for dysbindin. Hum Mol Genet 2009; 18: 2344-58. PMID: 19349376
- Ma X, Fei E, Fu C, Ren H, Wang G. Dysbindin-1, a schizophrenia-related protein, facilitates neurite outgrowth by promoting the transcriptional activity of p53. Mol Psychiatry. 2011; 16: 1105-16.PMID: 21502952
- Marley A, von Zastrow M. Dysbindin promotes the post-endocytic sorting of G protein-coupled receptors to lysosomes. PLoS One 2010; 5:e9325. PMID: 20174469
- Mead CL, Kuzyk MA, Moradian A, Wilson GM, Holt RA, Morin GB. Cytosolic protein interactions of the schizophrenia susceptibility gene dysbindin. J Neurochem. 2010; 113: 1491-503. PMID: 20236384
- Moriyama K, Bonifacino JS. Pallidin is a component of a multi-protein complex involved in the biogenesis of lysosome-related organelles. Traffic 2002; 3: 666-77. PMID: 12191018
- Murotani T, Ishizuka T, Hattori S, Hashimoto R, Matsuzaki S, Yamatodani A. High dopamine turnover in the brains of Sandy mice. Neurosci Lett 2007; 421: 47-51. PMID: 17548156
- Nagai T, Kitahara Y, Shiraki A, Hikita T, Taya S, Kaibuchi K, Yamada K. Dysfunction of dopamine release in the prefrontal cortex of dysbindin deficient sandy mice: an in vivo microdialysis study. Neurosci Lett 2010; 470: 134-8. PMID: 20045719
- Nazarian R, Starcevic M, Spencer MJ, Dell'angelica EC. Reinvestigation of the dysbindin subunit of BLOC-1 (biogenesis of lysosome-related organelles complex-1) as a dystrobrevin-binding protein. Biochem J 2006; 395: 587-98.PMID: 16448387
- Nian H, Fan C, Liao S, Shi Y, Zhang K, Liu Y, Han C. RNF151, a testis-specific RING finger protein, interacts with dysbindin. Arch Biochem Biophys 2007; 465: 157-63. PMID: 17577571
- Nihonmatsu-Kikuchi N, Hashimoto R, Hattori S, Matsuzaki S, Shinozaki T, Miura H, Ohota S, Tohyama M, Takeda M, Tatebayashi Y. Reduced rate of neural differentiation in the dentate gyrus of adult dysbindin null (sandy) mouse. PLoS One 2011; 6: e15886. PMID: 21267465
- Nguyen T, Novak EK, Kermani M, Fluhr J, Peters LL, Swank RT, Wei ML. Melanosome morphologies in murine models of hermansky-pudlak syndrome reflect blocks in organelle development. J Invest Dermatol 2002; 119: 1156-64. PMID: 12445206
- Numakawa T, Yagasaki Y, Ishimoto T, Okada T, Suzuki T, Iwata N, Ozaki N, Taguchi T, Tatsumi M, Kamijima K, Straub RE, Weinberger DR, Kunugi H, Hashimoto R.Evidence of novel neuronal functions of dysbindin, a susceptibility gene for schizophrenia. Hum Mol Genet. 2004;13: 2699-708. PMID: 15345706
- Ottis P, Bader V, Trossbach SV, Kretzschmar H, Michel M, Leliveld SR, Korth C. Convergence of Two Independent Mental Disease Genes on the Protein Level: Recruitment of Dysbindin to Cell-Invasive Disrupted-In-Schizophrenia 1 Aggresomes. Biol Psychiatry 2011; 70: 604-10.PMID: 21531389
- Oyama S, Yamakawa H, Sasagawa N, Hosoi Y, Futai E, Ishiura S. Dysbindin-1, a schizophrenia-related protein, functionally interacts with the DNA- dependent protein kinase complex in an isoform-dependent manner. PLoS ONE 2009; 4: e4199. PMID: 19142223
- Salazar G, Craige B, Styers ML, Newell-Litwa KA, Doucette MM, Wainer BH, Falcon-Perez JM, Dell'Angelica EC, Peden AA, Werner E, Faundez V. BLOC-1 complex deficiency alters the targeting of adaptor protein complex-3 cargoes. Mol Biol Cell 2006; 17: 4014-26. PMID: 16760431
- Setty SR, Tenza D, Truschel ST, Chou E, Sviderskaya EV, Theos AC, Lamoreux ML, Di Pietro SM, Starcevic M, Bennett DC, Dell'angelica EC, Raposo G, Marks MS. BLOC-1 Is Required for Cargo-specific Sorting from Vacuolar Early Endosomes toward Lysosome-related Organelles. Mol Biol Cell 2007; 18: 768-80. PMID: 17182842
- Sillitoe RV, Benson MA, Blake DJ, Hawkes R. Abnormal dysbindin expression in cerebellar mossy fiber synapses in the mdx mouse model of Duchenne muscular dystrophy. J Neurosci 2003; 23: 6576-85. PMID: 12878699
- Starcevic M, Dell'Angelica EC. Identification of snapin and three novel proteins (BLOS1, BLOS2, and BLOS3/reduced pigmentation) as subunits of biogenesis of lysosome-related organelles complex-1 (BLOC-1). J Biol Chem 2004; 279: 28393-401. PMID: 15102850
- Swank RT, Sweet HO, Davisson MT, Reddington M, Novak EK. Sandy: a new mouse model for platelet storage pool deficiency. Genet Res. 1991; 58: 51-62. PMID: 1936982
- Takao K, Toyama K, Nakanishi K, Hattori S, Takamura H, Takeda M, Miyakawa T, Hashimoto R. Impaired long-term memory retention and working memory in sdy mutant mice with a deletion in Dtnbp1, a susceptibility gene for schizophrenia. Mol Brain 2008; 1: 11. PMID: 18945333
- Talbot K, Cho DS, Ong WY, Benson MA, Han LY, Kazi HA, Kamins J, Hahn CG, Blake DJ, Arnold SE. Dysbindin-1 is a synaptic and microtubular protein that binds brain snapin. Hum Mol Genet 2006; 15: 3041-54. PMID: 16980328
- Taneichi-Kuroda S, Taya S, Hikita T, Fujino Y, Kaibuchi K. Direct interaction of Dysbindin with the AP-3 complex via its mu subunit. Neurochem Int 2009; 54: 431-8. PMID: 19428785
- Tang TT, Yang F, Chen BS, Lu Y, Ji Y, Roche KW, Lu B. Dysbindin regulates hippocampal LTP by controlling NMDA receptor surface expression. Proc Natl Acad Sci U S A. 2009; 106: 21395-400. PMID: 19955431
- Yin H, Laguna KA, Li G, Kuret J. Dysbindin structural homologue CK1BP is an isoform-selective binding partner of human casein kinase-1. Biochemistry 2006; 45: 5297-308. PMID: 16618118
EDIT HISTORY:
Created by Wei Li: 06/04/2004
Updated by Wei Li: 06/01/2005
Updated by Wei Li: 04/06/2006
Updated by Wei Li: 12/26/2006
Updated by Wei Li: 02/28/2008
Updated by Wei Li: 02/24/2009
Updated by Wei Li: 05/24/2011
Updated by Wei Li: 08/03/2012