GENOMIC
Mapping
3qC. View the map and BAC clones (data from UCSC genome browser).
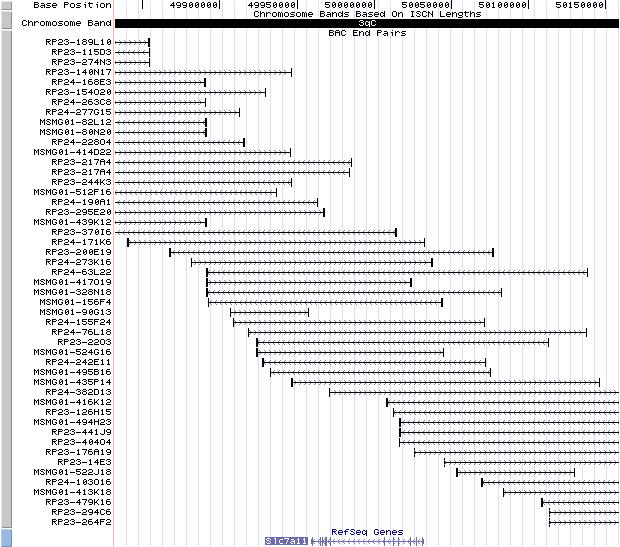
Structure
(assembly 03/2005)
Slc7a11/NM_011990: 12 exons, 72,621bp, chr3:49,959,006-50,031,626.
The figure below shows the structure of the Slc7a11 gene (data from UCSC genome browser).

Regulatory Element
Search the 5'UTR and 1kb upstream regions (seq1=human SLC7A11, seq2=mouse Slc7a11) by CONREAL with 80% Position Weight Matrices (PWMs) threshold (view results here). Two amino acid response elements (AAREs), each located in the opposite direction with an intervening sequence, were found in the 5'-flanking region of the mouse xCT gene (Sato, et al (2004)). The 5'-deletion analysis revealed that the sequence between -116 and -82 is essential for the basal expression and the sequence between -226 and -116 containing EpRE-1 is essential in response to diethyl maleate(Sasaki, et al).
TRANSCRIPT
RefSeq/ORF
Slc7a11 (NM_011990), 2,216bp, view ORF and the alignment to genomic.
Using 3'RACE of brain mRNA, two alternative transcripts with different polyA signals (AY766236) were identified, while in sut brain mRNA, an alternative exon 12' was identified (AY766237) (Chintala, et al ).
Expression Pattern
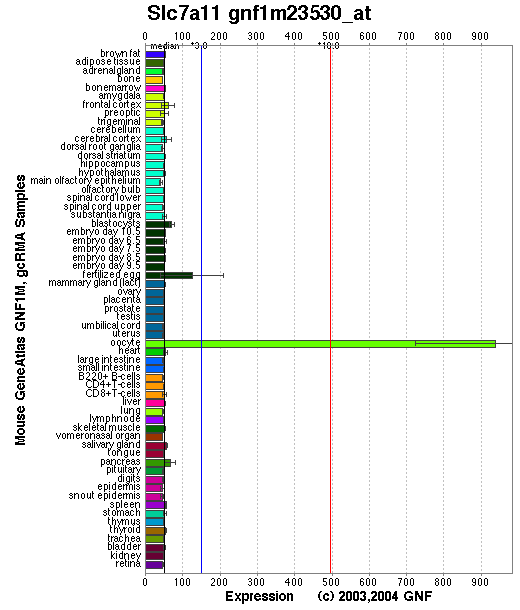
Tissue specificity: Widely expressed, highly expressed in brain and melanocytes. By in situ hybridization, the xCT mRNA was expressed in the area postrema, subfornical organ, habenular nucleus, hypothalamic area, and ependymal cells of the lateral wall of the third ventricle in the adult mouse brain. A strong signal was also detected in the meninges in both adult and fetal mouse brains (Sato, et al (2002)). Both xCT and 4F2hc were found to localize predominantly to neurons in the mouse and human brain, but some glial cells were labeled as well. Border areas between the brain proper and periphery including the vascular endothelial cells, ependymal cells, choroid plexus and leptomeninges were also highly positive for the system x(-)c components. xCT and 4F2hc are also present at the brush border membranes in the kidney and duodenum (Burdo, et al). In embryonic and adult rat brain, xCT protein was enriched at the CSF-brain barrier (i.e., meninges) and also expressed in the cortex, hippocampus, striatum, and cerebellum (Shih, et al).
Affymetrix microarray expression pattern in SymAtlas from GNF is shown below.
PROTEIN
Sequence
xCT (NP_036120): 502aa, UniProtKB/Swiss-Prot entry Q9WTR6.
Synonym: Amino acid transport system xc-, Cystine/glutamate transporter, solute carrier family 7 (cationic amino acid transporter, y+ system) member 11
Ortholog
| Species | Human | Rat | Dog | Fowl |
| GeneView | SLC7A11 | Slc7a11 | LOC483821 | LOC428731 |
| Protein | NP_055146 (501aa) | XP_227120 (502aa) | XP_540941 (551aa) | XP_426289 (580aa) |
| Identities | 89% /500aa | 99% /502aa | 91% /482aa | 71% /581aa |
View multiple sequence alignment (PDF file) by ClustalW and GeneDoc.
Domain
(1) Domains predicted by SMART:
AA_permease: 48- 482
(2) Transmembrane domains predicted by SOSUI:
This amino acid sequence is of a MEMBRANE PROTEIN which have 11 transmembrane helices.
| No. | N terminal | transmembrane region | C terminal | type | length |
| 1 | 44 | ITLLRGVSIIIGTVIGSGIFISP | 66 | SECONDARY | 23 |
| 2 | 75 | SVGMSLVFWSACGVLSLFGALSY | 97 | PRIMARY | 23 |
| 3 | 109 | GHYTYILEVFGPLLAFVRVWVEL | 131 | SECONDARY | 23 |
| 4 | 136 | PGATAVISLAFGRYILEPFFIQC | 158 | SECONDARY | 23 |
| 5 | 162 | ELAIKLVTAVGITVVMVLNSTSV | 184 | PRIMARY | 23 |
| 6 | 191 | QIFLTFCKLTAILIIIVPGVIQL | 213 | PRIMARY | 23 |
| 7 | 233 | MGLPLAFYYGMYAYAGWFYLNFI | 255 | SECONDARY | 23 |
| 8 | 267 | PLAICISMAIITVGYVLTNVAYF | 289 | PRIMARY | 23 |
| 9 | 390 | NFLSFARWLFMGLAVAGLIYLR | 411 | PRIMARY | 22 |
| 10 | 422 | KVPLFIPALFSFTCLFMVVLSLY | 444 | PRIMARY | 23 |
| 11 | 450 | TGVGFLITLTGVPAYYLFIVWD | 471 | SECONDARY | 22 |
Note: a topological model for xCT of 12 transmembrane domains with the N and C termini located inside the cell was proposed by Gasel et al .
(3) Graphic view of InterPro domain structure.
Motif/Site
(1) Predicted results by ScanProsite:
a) N-glycosylation site: 19 to 22 NMSG; 180 to 183 NSTS.
b) cAMP- and cGMP-dependent protein kinase phosphorylation site: 42 to 45 KKIT; 478 to 481 RRLS.
c) Protein kinase C phosphorylation site: 7 sites.
d) Casein kinase II phosphorylation site: 5 sites.
e) N-myristoylation site: 8 sites.
(2) Predicted results of subprograms by PSORT II:
a) N-terminal signal peptide: none
b) 11 tentative TMs, membrane topology: type 3a
c) KDEL ER retention motif in C-terminus: none
d) ER membrane retention signals:
XXRR-like motif in the N-terminus: VRKP; KKXX-like motif in the C-terminus: DSKE
e) VAC possible vacuolar targeting motif: none
f) Actinin-type actin-binding motif: type 1: none; type 2: none
g) Prenylation motif: none
h) memYQRL transport motif from cell surface to Golgi: none
i) Tyrosines in the tail: none
j) Dileucine motif in the tail: none
3D Model
(1)ModBase predicted comparative 3D structure of Q9WTR6 from UCSC Genome Sorter.
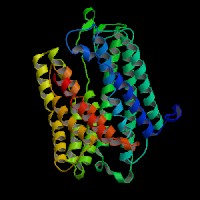
From left to right: Front, Top, and Side views of predicted protein.
(2) Mutation 3D modeling. Both C3H-xCT 3D and sut-xCT 3D structures are predicted by SPARKS and viewed by Protein Explorer.
2D-PAGE
This protein does not exist in the current release of SWISS-2DPAGE.
Computed theoretical MW=55,456Da, pI=9.31 (NP_036120).
FUNCTION
Ontology
(1) Biological process: amino acid transport.
(2) Pheomelanin production.
(3) Oxidative stress.
(4) Belongs to the amino acid-polyamine-organocation (APC) superfamily. L-type amino acid transporter (LAT) (TC 2.A.3.8) family.
Location
Integral membrane protein. Transport system xc- was found in plasma membrane of cultured mammalian cells (Sato, et al (1999)).
Interaction
SLC7A11 (xCT), together with SLC3A2 (4F2hc) linked by a disulfide bond at residue C158, encodes the heterodimeric amino acid transport system x(c)(-), which mediates cystine-glutamate exchange and thereby regulates intracellular glutathione levels. System x(c)- activity is important to maintain both intracellular glutathione levels and the redox balance between cystine and cysteine in the extracellular milieu. rBAT can replace 4F2hc in the expression of the activity of system x(c)(-) (Wang, et al).
In mouse NIH3T3 cells, the activity of system x(c)(-) and xCT mRNA is induced not only by deprivation of cystine but also by deprivation of other amino acids via a genomic cis-element termed amino acid response element (AARE) (Sato, et al (2004)). Overexpression of Nrf-2 in astrocytes specifically increases expression of the xCT cystine/glutamate antiporter and other thiol synthesis-related proteins, to promote cell survival (Qiang, et al). Cys(327) is a functionally important residue accessible to the aqueous extracellular environment and is structurally linked to the permeation pathway and/or the substrate binding site (Jimenez-Vidal, et al).
A CD44 variant (CD44v) interacts with xCT and controls the intracellular level of reduced glutathione (GSH). Ablation of CD44 induced loss of xCT from the cell surface and suppressed tumor growth in a transgenic mouse model of gastric cancer. It also induced activation of p38(MAPK), a downstream target of ROS, and expression of the gene for the cell cycle inhibitor p21(CIP1/WAF1)(Ishimoto, et al).
Kaposi's sarcoma-associated herpesvirus (KSHV, human herpesvirus 8) is the causative agent of Kaposi's sarcoma and other lymphoproliferative syndromes often associated with HIV/AIDS. xCT has been identified as a receptor mediating KSHV cell fusion (Kaleeba, et al).
miR-26b, which is down-regulated in human breast cancer specimens and cell lines, impairs viability and triggers apoptosis of human breast cancer MCF7 cells. SLC7A11 is identified as a direct target of miR-26b and its expression is remarkably increased in both breast cancer cell lines and clinical samples (Liu, et al (2011)).
Pathway
System x(c)(-) is a sodium-independent, high-affinity transporter of anionic amino acids with high specificity for anionic form of cystine and glutamate. Chintala, et al have reported that Slc7a11 regulates the pheomelanin production and cell proliferation. In melanosomes, dopaquinone reacts with cysteine to yield cysteinldopa, which is a precursor of pheomelanin. Cysteine is a component of the tripeptide glutathione. Glutathione metabolism pathway is described in KEGG Pathways.
MUTATION
Allele or SNP
1 phenotypic allele is described in MGI:1857061.
SNPs deposited in dbSNP.
Distribution
| Location | Genomic | cDNA | Protein | Type | Strain | Reference |
| Intron 11 | del 481,280 bp | del exon 12 | D482-L502 del | gross deletion | sut (C3H/HeSnJ) | Chintala, et al |
Effect
The deletion creates a new splice site and replacement of exon 12 with exon 12'(sequence is shown in AY766237 ). A new stop codon in exon 12' is predicted to produce a modified and trancated carboxyl terminus (482AYYPPEKEG490). In Northern blots, the normal 9kb (brain) or 9.5kb (melanocytes) transcripts are markedly deficient, which reveals a possible null mutation in sut mice.(Chintala, et al).
PHENOTYPE
Mutation in the Slc7a11 gene is the cause of recessive subtle gray (sut) mutant (Chintala, et al). The sut allele arose spontaneously in the C3H/HeSnJ strain. The strain is described in more detail in JAX Mice database
(C3H/HeSnJ-sut/J).
Homozygotes have diluted coat color, with which yellow hair pigment is reduced, but black pigment is unaffected (Lane, et al). The sut mouse is also a model for a mild form of Hermansky-Pudlak syndrome, in which bleeding times of mutant mice were significantly prolonged (3.4-fold) in comparison with those in normal sut/+ controls (Swank, et al).
Chintala, et al reported that transport of cystine by xCT is critical for normal cultured cell growth, glutathione production, and protection from oxidative stress in cultured cells. Similarly, in the xCT-knock out (KO) mice, the embryonic fibroblasts failed to survive in routine culture medium (Sato, et al (2005)). Qiao, et al has demonstrated that the death of cultured sut cells is caused by apoptosis mediated by JNK activation with the involvement of both oxidative stress and ER stress.
The sut mice developed brain atrophy by early adulthood, exhibiting ventricular enlargement, thinning of the cortex, and shrinkage of the striatum. Astrocytes and meningeal cells derived from sut/sut mice showed greatly reduced proliferation in culture attributable to increased oxidative stress and thiol deficiency (Shih, et al). Deletion of xCT did not result in decreased glutathione levels in striatum. Accordingly, no signs of increased oxidative stress could be observed in striatum or substantia nigra of xCT(-/-) mice. However, xCT(-/-) mice were less susceptible to 6-hydroxydopamine (6-OHDA)-induced neurodegeneration in the substantia nigra pars compacta compared to their age-matched wild-type littermates. This reduced sensitivity to a PD-inducing toxin might be related to the decrease of 70% in striatal extracellular glutamate levels that was observed in mice lacking xCT (Massie, et al). xCT(-/-) mice do not have a lower hippocampal glutathione content, increased oxidative stress or brain atrophy, nor exacerbated spatial reference memory deficits with aging. Together, loss of system x(c)(-) does not induce oxidative stress in vivo. Young xCT(-/-) mice did however display a spatial working memory deficit. In contrast, significantly lower extracellular hippocampal glutamate concentrations in xCT(-/-) mice were measures compared to wild-type littermates. Correspondingly, xCT deletion in mice elevated the threshold for limbic seizures and abolished the proconvulsive effects of N-acetylcysteine (De Bundel, et al). Interestingly, rates of cell proliferation in the denate gyrus (DG) but not in the subventricular zone (SVZ) were elevated in both 3- and 11-month-old sut mice compared to the controls (Liu, et al (2007)).
REFERENCE
- Burdo J, Dargusch R, Schubert D. Distribution of the Cystine/Glutamate Antiporter System x-c in the Brain, Kidney, and Duodenum. J Histochem Cytochem 2006; 54: 549-57. PMID: 16399997
- Chintala S, Li W, Lamoreux ML, Ito S, Wakamatsu K, Sviderskaya EV, Bennett DC, Park YM, Gahl WA, Huizing M, Spritz RA, Ben S, Novak EK, Tan J, Swank RT. Slc7a11 gene controls production of pheomelanin pigment and proliferation of cultured cells. Proc Natl Acad Sci U S A 2005; 102: 10964-9. PMID: 16037214
- De Bundel D, Schallier A, Loyens E, Fernando R, Miyashita H, Van Liefferinge J, Vermoesen K, Bannai S, Sato H, Michotte Y, Smolders I, Massie A. Loss of System xFormula Does Not Induce Oxidative Stress But Decreases Extracellular Glutamate in Hippocampus and Influences Spatial Working Memory and Limbic Seizure Susceptibility. J Neurosci 2011; 31: 5792-803. PMID: 21490221
- Gasol E, Jimenez-Vidal M, Chillaron J, Zorzano A, Palacin M. Membrane topology of system xc- light subunit reveals a re-entrant loop with substrate-restricted accessibility. J Biol Chem 2004; 279:31228-36. PMID: 15151999
- Ishimoto T, Nagano O, Yae T, Tamada M, Motohara T, Oshima H, Oshima M, Ikeda T, Asaba R, Yagi H, Masuko T, Shimizu T, Ishikawa T, Kai K, Takahashi E, Imamura Y, Baba Y, Ohmura M, Suematsu M, Baba H, Saya H. CD44 variant regulates redox status in cancer cells by stabilizing the xCT subunit of system xc(-) and thereby promotes tumor growth. Cancer Cell 2011; 19: 387-400. PMID: 21397861
- Jimenez-Vidal M, Gasol E, Zorzano A, Nunes V, Palacin M, Chillaron J. Thiol modification of cysteine 327 in the eighth transmembrane domain of the light subunit xCT of the heteromeric cystine/glutamate antiporter suggests close proximity to the substrate binding site/permeation pathway. J Biol Chem 2004; 279: 11214-21. PMID: 14722095
- Kaleeba JA, Berger EA. Kaposi's sarcoma-associated herpesvirus fusion-entry receptor: cystine transporter xCT. Science 2006; 311: 1921-4. PMID: 16574866
- Lane PW, [Subtle gray (sut); small with kinky tail (skt)]. Mouse News Lett 1988; 80:165. No Abstract.
- Liu RR, Brown CE, Murphy TH. Differential regulation of cell proliferation in neurogenic zones in mice lacking cystine transport by xCT.Biochem Biophys Res Commun 2007; 364: 528-33. PMID: 17963724
- Liu XX, Li XJ, Zhang B, Liang YJ, Zhou CX, Cao DX, He M, Chen GQ, He JR, Zhao Q. MicroRNA-26b is underexpressed in human breast cancer and induces cell apoptosis by targeting SLC7A11. FEBS Lett 2011; 585: 1363-7. PMID: 21510944
- Massie A, Schallier A, Kim SW, Fernando R, Kobayashi S, Beck H, De Bundel D, Vermoesen K, Bannai S, Smolders I, Conrad M, Plesnila N, Sato H, Michotte Y. Dopaminergic neurons of system x(c)?-deficient mice are highly protected against 6-hydroxydopamine-induced toxicity. FASEB J 2011; 25: 1359-69. PMID: 21191088
- Qiang W, Cahill JM, Liu J, Kuang X, Liu N, Scofield VL, Voorhees JR, Reid AJ, Yan M, Lynn WS, Wong PK. Activation of transcription factor Nrf-2 and its downstream targets in response to moloney murine leukemia virus ts1-induced thiol depletion and oxidative stress in astrocytes. J Virol 2004; 78: 11926-38. PMID: 15479833
- Qiao HX, Hao CJ, Li Y, He X, Chen RS, Cui J, Xu ZH, Li W. JNK activation mediates the apoptosis of xCT-deficient cells. Biochem Biophys Res Commun 2008; 370: 584-8. PMID: 18395005
- Sasaki H, Sato H, Kuriyama-Matsumura K, Sato K, Maebara K, Wang H, Tamba M, Itoh K, Yamamoto M, Bannai S. Electrophile response element-mediated induction of the cystine/glutamate exchange transporter gene expression. J Biol Chem 2002; 277: 44765-71. PMID: 12235164
- Sato H, Tamba M, Ishii T, Bannai S. Cloning and expression of a plasma membrane cystine/glutamate exchange transporter composed of two distinct proteins. J Biol Chem 1999; 274:11455-8. PMID: 10206947
- Sato H, Tamba M, Okuno S, Sato K, Keino-Masu K, Masu M, Bannai S. Distribution of cystine/glutamate exchange transporter, system x(c)-, in the mouse brain. J Neurosci 2002; 22: 8028-33.PMID: 12223556
- Sato H, Nomura S, Maebara K, Sato K, Tamba M, Bannai S. Transcriptional control of cystine/glutamate transporter gene by amino acid deprivation. Biochem Biophys Res Commun 2004; 325: 109-16. PMID: 155522208
- Sato H, Shiiya A, Kimata M, Maebara K, Tamba M, Sakakura Y, Makino N, Sugiyama F, Yagami K, Moriguchi T, Takahashi S, Bannai S. Redox imbalance in cystine/glutamate transporter-deficient mice. J Biol Chem 2005; 280: 37423-9. PMID: 16144837
- Shih AY, Erb H, Sun X, Toda S, Kalivas PW, Murphy TH. Cystine/glutamate exchange modulates glutathione supply for neuroprotection from oxidative stress and cell proliferation. J Neurosci 2006; 26: 10514-23. PMID: 17035536
- Swank RT, Reddington M, Novak EK. Inherited prolonged bleeding time and platelet storage pool deficiency in the subtle gray (sut) mouse. Lab Anim Sci 1996; 46:56-60.PMID: 8699821
- Wang H, Tamba M, Kimata M, Sakamoto K, Bannai S, Sato H. Expression of the activity of cystine/glutamate exchange transporter, system x(c)(-), by xCT and rBAT. Biochem Biophys Res Commun 2003; 305: 611-8. PMID: 12763038
EDIT HISTORY:
Created by Wei Li, 08/12/2005
Updated by Wei Li, 04/06/2006
Updated by Wei Li, 12/28/2006
Updated by Wei Li, 02/28/2008
Updated by Wei Li, 08/20/2008
Updated by Wei Li, 05/29/2011