GENOMIC
Mapping
2qE5. View the map and BAC contig (data from UCSC genome browser).
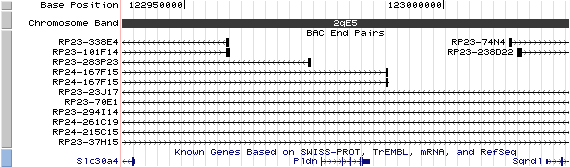
Structure
(assembly 10/03)
Pldn/NM_019788: 5 exons, 9,586bp, Chr2: 122,976,215-122,985,800.
The figure below shows the structure of the Pldn gene (data from UCSC genome browser).

Regulatory Element
Search the 5'UTR and 1kb upstream regions (human and mouse) by CONREAL with 80% Position Weight Matrices (PWMs) threshold (view results here).
TRANSCRIPT
RefSeq/ORF
Pldn (NM_019788), 2,580bp, view ORF and the alignment to genomic.
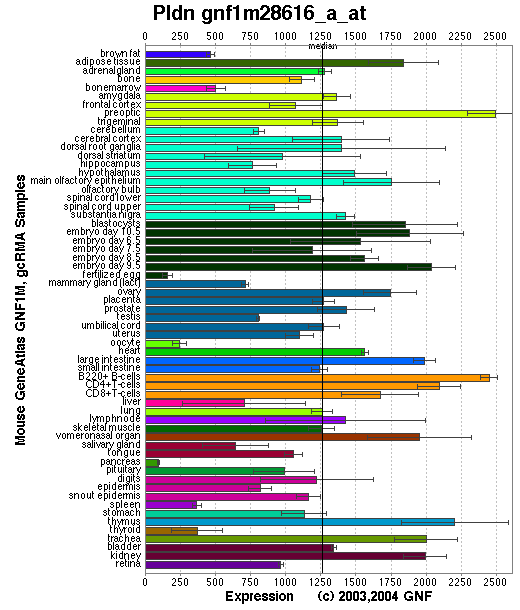
Expression Pattern
Tissue specificity: Ubiquitously expressed, with the highest expression levels observed in brain, heart, liver and kidney. A ~2.37kb band is present on the Northern blot (Huang, et al).
Affymetrix microarray expression pattern in SymAtlas from GNF is shown below.
PROTEIN
Sequence
Pallidin (NP_062762): 172aa, ExPaSy NiceProt view of Swiss-Prot:Q9R0C0.
Synonyms: Syntaxin 13 binding protein 1 (Stx13bp1); Syntaxin 13 interacting protein; Pallid protein.
Ortholog
| Species | Human | Chimp | Rat | Drosophila |
| GeneView | PLDN | 07038 | LOC317630 | CG14133 |
| Protein | NP_036520 (172aa) | 12031 (172aa) | XP_229231 (692aa) | NP_648494 (120aa) |
| Identities | 87%/149aa | 87%/149aa | 94%/162aa | 32%/36aa |
View multiple sequence alignment (PDF file) by ClustalW and GeneDoc.
Domain
(1) Domains predicted by SMART:
a) coiled coil: 68 - 88.
b) coiled coil: 125 - 167.
(2) Transmembrane domains predicted by SOSUI: None.
Motif/Site
(1) Predicted results by ScanProsite:
a) Casein kinase II phosphorylation site : [occurs frequently]
2 - 5: SvpE,
32 - 35: TspD,
33 - 36: SpdE,
97 - 100: SmlD.
b) N-myristoylation site : [occurs frequently]
28 - 33: GLsdTS,
54 - 59: GGllSH.
c) Protein kinase C phosphorylation site : [occurs frequently]
67 - 69: SkR,
117 - 119: TiR,
129 - 131: TsK,
165 - 167: TaK.
d) Bipartite nuclear targeting sequence : [occurs frequently]
119 - 135:
RKemlllhektsklkkr.
(2) Predicted results of subprograms by PSORT II:
a) N-terminal signal peptide: none
b) KDEL ER retention motif in the C-terminus: none
c) ER Membrane Retention Signals: found KKXX-like motif in the C-terminus: PAKR
d) VAC possible vacuolar targeting motif: none
e) Actinin-type actin-binding motif: type 1: none; type 2: none
f) Prenylation motif: none
g) memYQRL transport motif from cell surface to Golgi: none
h) Tyrosines in the tail: none
i) Dileucine motif in the tail: None.
3D Model
(1) ModBase No entry found.
(2) 3D models predicted by SPARKS (fold recognition) below. View the models by PDB2MGIF.
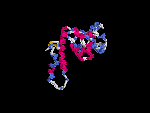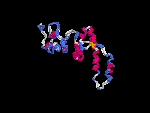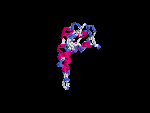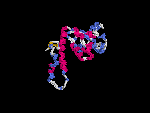
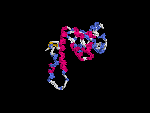
2D-PAGE
This protein does not exist in the current release of SWISS-2DPAGE.
Computed theoretical MW=19,682Da, pI=5.91 (NP_062762).
FUNCTION
Ontology
(1) Process: pigmentation, vesicle-mediated transport.
(2) Protein interaction in BLOC-1.
(3) May play a role in intracellular vesicle trafficking (view diagram of trafficking pathway here), particularly in the vesicle-docking and fusion process.
Location
Cytoplasmic. It can exist as a soluble protein as well as a peripheral membrane protein.
Interaction
Pallidin interacts with muted, cno, dysbindin, Blos1, syntaxin 13, and F-actin (Ciciotte, et al; Falcon-Perez, et al; Li, et al (2004); Starcevic, et al). Pallidin is a subunit of the biogenesis of lysosome-related organelles complex 1 (BLOC-1), which contains the products of seven other HPS genes, sdy, mu, cno, rp, Snapap, Blos1, Blos2 (Ciciotte, et al; Falcon-Perez , et al; Li, et al (2003); Moriyama, et al; Starcevic, et al) (view diagram of BLOC-1 complex here). Syntaxin-13 is a member of t-SNARE which is localized to the endosomal membrane and is required for vesicle fusion to the early enddosomes and recycling endosomes. Yeast two-hybrid analyses revealed that pallidin is capable of self-association through a region that contains its two coiled-coil forming domains. Immunofluorescence and in vitro binding experiments demonstrated that pallidin/BLOC-1 is able to associate with actin filaments ( Falcon-Perez, et al ). More details about the function of BLOC-1 are described in the HPS7 profile.
Pallidin drosophila homolog CG14133 interaction information in CuraGen interaction database.
Pathway
Involved in the development of lysosome-related organelles, such as melanosomes and platelet-dense granules (view diagram of BLOC-1 pathway here). Nguyen, et al found that the greatest percentages of immature melanosomes were observed in the BLOC-1 mutants pa and cno, which suggests the maturation of melanosome is blocked between the multivesicular body stage and stage I (view diagram of melanosome blockage here).
MUTATION
Allele or SNP
1 phenotypic allele is described in MGI:1927580.
Distribution
| Location | Genomic | cDNA | Protein | Type | Strain | Reference |
| Exon 2 | 205C>T | 205C>T | R69X | nonsense | pa (B6) | Huang, et al |
Effect
The nonsense mutation leads to exon 2 skipping, which causes a truncated protein. By Western blot analysis, the pallidin is undetectable in pa mutants (Huang, et al; Li, et al (2003) ). The mutation does affect the stability of other subunits such as dysbindin and muted of BLOC-1 complex (Li, et al (2003)).
PHENOTYPE
The pallid mouse is an appropriate mouse model of Hermansky-Pudlak syndrome (OMIM 604310), mimicking the HPS-9 phenotypes (Cullinane, et al) . The pa allele was made congenic by backcrossing with C57BL/6J. The mutant is described in more detail in JAX Mice database (B6.Cg-Pldnpa/J). Defects in Pldn gene are the cause of the pallid (pa) phenotype (Huang, et al) that is characterized by an altered formation or function of intracellular storage granules in melanocytes, platelets, and lysosomes in kidney. Pallid mice have a prolonged bleeding time owing to the inability of immature platelet dense granules to accumulate normal amounts of ATP, ADP, and serotonin. Pa animals also suffer from pigment dilution, kidney lysosomal enzyme elevation and serum alpha1-antitrypsin activity deficiency. Finally, pallid mice exhibit defects in otolith formation that lead to balance abnormalities (Novak, et al). The phenotype is described in more detail in Mouse Locus Catalog#Pldn.
REFERENCE
- Ciciotte SL, Gwynn B, Moriyama K, Huizing M, Gahl WA, Bonifacino JS, Peters LL. Cappuccino, a mouse model of Hermansky-Pudlak syndrome, encodes a novel protein that is part of the pallidin-muted complex (BLOC-1). Blood 2003; 101: 4402-7. PMID: 12576321
- Cullinane AR, Curry JA, Carmona-Rivera C, Summers CG, Ciccone C, Cardillo ND, Dorward H, Hess RA, White JG, Adams D, Huizing M, Gahl WA. A BLOC-1 Mutation Screen Reveals that PLDN Is Mutated in Hermansky-Pudlak Syndrome Type 9. Am J Hum Genet 2011; 88: 778-87. PMID: 21665000
- Falcon-Perez JM, Starcevic M, Gautam R, Dell'Angelica EC. BLOC-1, a novel complex containing the pallidin and muted proteins involved in the biogenesis of melanosomes and platelet-dense granules. J Biol Chem 2002; 277: 28191-9. PMID: 12019270
- Huang L, Kuo YM, Gitschier J. The pallid gene encodes a novel, syntaxin 13-interacting protein involved in platelet storage pool deficiency. Nat Genet 1999; 23: 329-32. PMID: 10610180
- Li W, Rusiniak ME, Chintala S, Gautam R, Novak EK, Swank RT. Murine Hermansky-Pudlak syndrome genes: regulators of lysosome-related organelles. Bioessays 2004; 26: 616-28. PMID: 15170859
- Li W, Zhang Q, Oiso N, Novak EK, Gautam R, O'Brien EP, Tinsley CL, Blake DJ, Spritz RA, Copeland NG, Jenkins NA, Amato D, Roe BA, Starcevic M, Dell'Angelica EC, Elliott RW, Mishra V, Kingsmore SF, Paylor RE, Swank RT. Hermansky-Pudlak syndrome type 7 (HPS-7) results from mutant dysbindin, a member of the biogenesis of lysosome-related organelles complex 1 (BLOC-1). Nat Genet 2003; 35: 84-9. PMID: 12923531
- Moriyama K, Bonifacino JS. Pallidin is a component of a multi-protein complex involved in the biogenesis of lysosome-related organelles. Traffic 2002; 3: 666-77. PMID: 12191018
- Nguyen T, Novak EK, Kermani M, Fluhr J, Peters LL, Swank RT, Wei ML. Melanosome morphologies in murine models of hermansky-pudlak syndrome reflect blocks in organelle development. J Invest Dermatol 2002; 119: 1156-64. PMID: 12445206
- Novak EK, Hui SW, Swank RT. Platelet storage pool deficiency in mouse pigment mutations associated with seven distinct genetic loci. Blood 1984; 63: 536-44. PMID: 6696991
- Starcevic M, Dell'Angelica EC. Identification of snapin and three novel proteins (BLOS1, BLOS2, and BLOS3/reduced pigmentation) as subunits of biogenesis of lysosome-related organelles complex-1 (BLOC-1). J Biol Chem 2004; 279: 28393-401. PMID: 15102850
EDIT HISTORY:
Created by Wei Li & Jonathan Bourne, 07/08/2004
Updated by Wei Li, 08/03/2012