GENOMIC
Mapping
10qC1 View the map and BAC contig (data from UCSC genome browser).
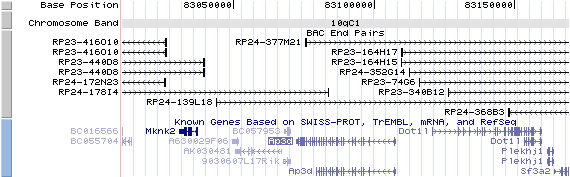
Structure
(assembly 10/03)
Ap3d/NM_007460: 31 exons, 35,235bp, Chr10: 83,072,201 - 83,107,435.
The figure below shows the map of the known isoform (data from UCSC genome browser).

Regulatory Element
Search the 5'UTR and 1kb upstream regions (human and mouse) by CONREAL with 80% Position Weight Matrices (PWMs) threshold (view results here).
TRANSCRIPT
RefSeq/ORF
Ap3d (NM_007460),
4,730bp, view ORF and the alignment to genomic..
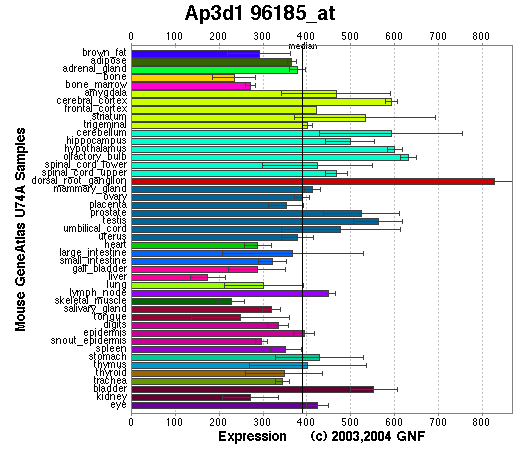
Expression Pattern
Tissue specificity: Varied.
Affymetrix microarray expression pattern in SymAtlas from GNF is shown below.
PROTEIN
Sequence
AP-3 complex delta subunit
(NP_031486): 1199aa, ExPaSy NiceProt view of Swiss-Prot:O54774.
Synonyms: Adaptor-related protein complex 3, delta subunit.
Ortholog
| Species | Human | Rat | Zebrafish | Worm | Drosophila |
| GeneView | AP3D1 | LOC314633 | 03334 | apt-5 | g/CG10986 |
| Protein | NP_003929 (1138aa) | XP_234908 (1204aa) | 17714 (966aa) | NP_494570 (1251aa) | NP_524785 (1034aa) |
| Identities | 86%/1014aa | 97%/1177aa | 79%/763aa | 45%/580aa | 55%/560aa |
View multiple sequence alignment (PDF file) by ClustalW and GeneDoc.
Domain
(1) Domains predicted by SMART:
a) low complexity: 629 - 642.
b) Pfam domains:
PF01602 Adaptin N terminal region: 32 - 583.
PF06375 BLVR: 644-1197.
(2) Transmembrane domains predicted by SOSUI:
1 transmembrane helix, 272-294.
(3) CDD conserved domain: KOG1059, vesicle coat complex AP-3, delta subunit (Intracellular trafficking, secretion, and vesicular transport).
(4) Graphic view of InterPro domain structure.
Motif/Site
(1) Predicted results by ScanProsite:
a) Lysine-rich region profile : [occurs frequently]
836 - 944: score=11.364
b) Tyrosine kinase phosphorylation site : [occurs frequently]
29 - 35: Ked.Eak.Y,
359 - 365: Ral.Dll.Y
c) N-myristoylation site : [occurs frequently]
129 - 134: GValTG,
198 - 203: GVqsAA,
430 - 435: GTrhGH,
589 - 594: GVpvAE,
717 - 722: GMpmSD,
1122 - 1127: GIsmSF.
d) Amidation site : [occurs frequently]
255 - 258: lGKK,
748 - 751: kGKR.
e) N-glycosylation site : [occurs frequently]
296 - 299: NHSA,
1001 - 1004: NQSS.
f) cAMP- and cGMP-dependent protein kinase phosphorylation site : [occurs frequently]
751 - 754: RRhS,
915 - 918: RKsS.
g) Tyrosine sulfation site : [occurs frequently]
385 - 399:
dkaegttYrdelltk,
717 - 731:
gmpmsdqYvkleeqr,
788 - 802:
dkdpndpYraldidl.
(2) Predicted results of subprograms by PSORT II:
a) N-terminal signal peptide: none
b) KDEL ER retention motif in the C-terminus: none
c) ER Membrane Retention Signals: none
d) VAC possible vacuolar targeting motif: none
e) Actinin-type actin-binding motif: type 1: none; type 2: none
f) Prenylation motif: none
g) memYQRL transport motif from cell surface to Golgi: none
h) Tyrosines in the tail: too long of a tail
i) Dileucine motif in the tail: found LL at 1184, 1188.
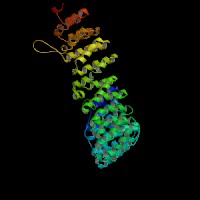
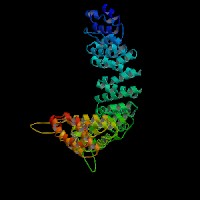
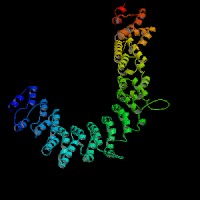
3D Model
(1) ModBase: 1 match found, results here.
(2) ModBase predicted comparative 3D structures on O54774 (data from UCSC Gene Sorter). (from left to right: Front, Top, Side view)
2D-PAGE
This protein does not exist in the current release of SWISS-2DPAGE.
Computed theoretical MW=135,081Da, pI=7.04 (NP_031486).
FUNCTION
Ontology
a) Biological process: intracellular protein transport, vesicle-mediated transport (overview of trafficking pathway here).
b) Component of Golgi trans face.
Location
Component of the coat surrounding the cytoplasmic face of coated vesicles located at the Golgi complex.
Interaction
Ap3d gene encodes the adaptor-related protein complex AP-3, delta subunit. The AP-3 complex is a heterotetramer composed of two large adaptins (delta/AP3D1 and beta3A/AP3B2 or beta3B/AP3B1), a medium adaptin (mu3A/AP3M1 or mu3B/AP3M2) and a small adaptin (sigma3A/AP3S1 or sigma3B/AP3S2). AP-3, has been identified that interacts with dileucine motifs and mediates endosomal/lysosomal transport in yeast, drosophila, and mammals. In addition, the AP3M1 subunit interacts with tyrosinase for lysosomal targeting (Honing, et al). AP-3 complex interacts with CD1 antigen presenting molecules. In mocha cells, the beta3 and mu3 subunits coassemble into a heterodimer, whereas the sigma3 subunit remains monomeric (Peden, et al).
6 proteins are shown to be associated with APL5 in Yeast GRID.
Ap3d drosophila homolog CG10986 interaction information in CuraGen interaction database.
Pathway
AP-3 complex is associated with the Golgi region as well as more peripheral structures. It facilitates the budding of vesicles from the Golgi membrane and appears to be involved in the sorting of a subset of transmembrane proteins targeted to lysosomes and lysosome-related organelles. In yeast, AP-3 sorts the vacuolar membrane enzymes, alkaline phosphatase and Vamp3p, a vacuolar t-SNARE (Odorizzi, et al).
In neurons, one cargo of the AP-3 complex is zinc transporter ZnT-3. Blumstein, et al proposed that the neuronal form of AP-3, which is required for synaptic vesicle formation, rather than the ubiquitous form may be responsible for the neurological abnormalities. Faundez, et al found that the AP-3 complex is involved in synaptic vesicle formation in neuronal cells. Likewise, Sugita, et al concluded that there is an AP-3 dependent pathway for antigen presentation by CD1B (human) or CD1d (mice) molecules. The mechanism of AP-3 dependent pathway is distinct from that dependent on HPS1 (Feng, et al) (view diagram of BLOC-3 and AP-3 pathway here).
AP-3 complex is associated with the Golgi region as well as more peripheral structures. It facilitates the budding of vesicles from the Golgi membrane and appears to be involved in the sorting of a subset of transmembrane proteins targeted to lysosomes and lysosome-related organelles. In yeast, AP-3 sorts the vacuolar membrane enzymes, alkaline phosphatase and Vamp3p, a vacuolar t-SNARE (Odorizzi, et al).
In neurons, one cargo of the AP-3 complex is zinc transporter ZnT-3. Blumstein, et al proposed that the neuronal form of AP-3, which is required for synaptic vesicle formation, rather than the ubiquitous form may be responsible for the neurological abnormalities. Faundez, et al found that the AP-3 complex is involved in synaptic vesicle formation in neuronal cells. Likewise, Sugita, et al concluded that there is an AP-3 dependent pathway for antigen presentation by CD1B (human) or CD1d (mice) molecules. The mechanism of AP-3 dependent pathway is distinct from that dependent on HPS1 (Feng, et al) (view diagram of BLOC-3 and AP-3 pathway here).
MUTATION
Allele or SNP
2 phenotypic alleles are described in MGI:107734.
SNPs deposited in dbSNP.
Distribution
| Location | Genomic | cDNA | Protein | Type | Strain | Reference |
| Intron 1 | del ~12kb | G97~G592del 496bp (exon 2~exon 6) | A33 del 496bp | frame-shift, 64X | mh (B6) | Kantheti, et al (1998) |
| Intron 21 | intron 21 +5Gins 5~6kb IAP, insGGTGAG | 2797Gins 2kb IAP element | K859ins 2kb | gross insertion | mh-2J (C3H/HeJ) | Kantheti, et al (2003) |
Effect
mocha is a null allele of the subunit of the adaptor-like protein complex AP-3. The C-terminal truncated delta subunit in mh2J is stable and able to correctly assemble and localize the AP-3 complex to the TGN and endosomes.
PHENOTYPE
Defects in AP3d are the cause of the autosomal recessive phenotype 'mocha' (mh), a mouse model of Hermansky-Pudlak syndrome (Swank, et al). The mh allele arose spontaneously from C57BL/6J-pi. The strain is described in more detail in JAX Mice database (Ap3dmh/J). Similar to muted mice, the mocha mice also have inner ear abnormalities (balance defects due to otolith defects), prolonged bleeding times accompanied by abnormalities of dense granules as determined by whole mount electron microscopy of platelets and by labeling platelets with mepacrine. When mutant platelets were treated with collagen, there was minimal secretion of adenosine triphosphate and aggregation was reduced. Lysosomal enzyme secretion in response to thrombin treatment was markedly reduced in mocha platelets. Similar reductions in constitutive lysosomal enzyme secretion from kidney proximal tubule cells were noted in the mutants. Mocha mice have hearing loss progressing to complete deafness by 3-6 months of age(Swank, et al). In addition, the mocha mutation modulates the synchronous synaptic activation of neocortical neurons, resulting in a constant 6-7 Hz (theta) wave pattern in the electrocorticogram. See more in details in Mouse Locus Card#Ap3d.
Mice mutant for a second allele, mh2J, a hypomorph of mh, are less severely affected and have a different cortical excitability phenotype, with no persistent hypersynchronization but episodes of spike-wave and tonic clonic seizures (Noebels, et al). In mocha brain, the ZnT-3 transporter is reduced, resulting in a lack of zinc-associated Timm historeactivity in hippocampal mossy fibers (Kantheti, et al (1998)). In contrast to mh, the mh2J brain reveals a normal distribution of ZnT-3 in hippocampal mossy fibers, but abnormal ZnT-3 accumulation in the soma of neurons and loss of vesicular zinc staining in neocortical synapses (Kantheti, et al (2003)).
REFERENCE
- Blumstein J, Faundez V, Nakatsu F, Saito T, Ohno H, Kelly RB. The neuronal form of adaptor protein-3 is required for synaptic vesicle formation from endosomes. J Neurosci 2001; 21: 8034-42. PMID: 11588176
- Faundez V, Horng JT, Kelly RB. A function for the AP3 coat complex in synaptic vesicle formation from endosomes. Cell 1998; 93: 423-32. PMID: 9590176
- Feng L, Novak EK, Hartnell LM, Bonifacino JS, Collinson LM, Swank RT. The Hermansky-Pudlak syndrome 1 (HPS1) and HPS2 genes independently contribute to the production and function of platelet dense granules, melanosomes, and lysosomes. Blood 2002; 99: 1651-8. PMID: 11861280
- Honing S, Sandoval IV, von Figura K. A di-leucine-based motif in the cytoplasmic tail of LIMP-II and tyrosinase mediates selective binding of AP-3. EMBO J 1998; 17: 1304-14. PMID: 9482728
- Kantheti P, Diaz ME, Peden AE, Seong EE, Dolan DF, Robinson MS, Noebels JL, Burmeister ML. Genetic and phenotypic analysis of the mouse mutant mh2J, an Ap3d allele caused by IAP element insertion. Mamm Genome 2003; 14: 157-67. PMID: 12647238
- Kantheti P, Qiao X, Diaz ME, Peden AA, Meyer GE, Carskadon SL, Kapfhamer D, Sufalko D, Robinson MS, Noebels JL, Burmeister M. Mutation in AP-3 delta in the mocha mouse links endosomal transport to storage deficiency in platelets, melanosomes, and synaptic vesicles. Neuron 1998; 21: 111-22. PMID: 9697856
- Noebels JL, Sidman RL. Persistent hypersynchronization of neocortical neurons in the mocha mutant of mouse. J Neurogenet 1989; 6: 53-6. PMID: 2778559
- Odorizzi G, Cowles CR, Emr SD. The AP-3 complex: a coat of many colours. Trends Cell Biol 1998; 8: 282-8. PMID: 9714600
- Peden AA, Rudge RE, Lui WW, Robinson MS. Assembly and function of AP-3 complexes in cells expressing mutant subunits. J Cell Biol 2002; 156: 327-36. PMID: 11807095
- Sugita M, Cao X, Watts GF, Rogers RA, Bonifacino JS, Brenner MB. Failure of trafficking and antigen presentation by CD1 in AP-3-deficient cells. Immunity 2002; 16: 697-706. PMID: 12049721
- Swank RT, Reddington M, Howlett O, Novak EK. Platelet storage pool deficiency associated with inherited abnormalities of the inner ear in the mouse pigment mutants muted and mocha. Blood 1991; 78: 2036-44. PMID: 1912584
EDIT HISTORY:
Created by Wei Li: 07/02/2004