GENOMIC
Mapping
7qD3. View the map and BAC clones (data from UCSC genome browser).
Structure
(assembly 10/03)
Rab38/NM_028238: 3 exons, 61,300bp, Chr7: 80,826,675-80,887,974.
The figure below shows the structure of the Rab38 gene (data from UCSC genome browser).
Regulatory Element
Search the 5'UTR and 1kb upstream regions (seq1=human RAB38, seq2=mouse Rab38) by CONREAL with 80% Position Weight Matrices (PWMs) threshold (view results here).
TRANSCRIPT
RefSeq/ORF
Rab38 (NM_028238), 1,612bp, view ORF and the alignment to genomic.
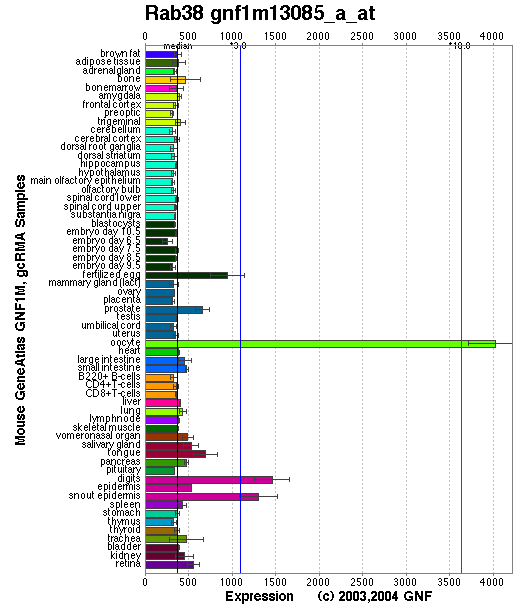
Expression Pattern
Tissue specificity: Mouse Rab38 transcript is detected in melanocytes, melanoma cell lines, lung type II cells, and at varying levels in many tissues including adrenal gland, uterus, testes, kidney, prostate, placenta, and others.
Affymetrix microarray expression pattern in SymAtlas from GNF is shown below.
PROTEIN
Sequence
Rab38 (NP_082514): 211aa, ExPaSy NiceProt view of Swiss-Prot:Q8QZZ8.
Synonym: Ras-related protein Rab-38
Ortholog
| Species | Human | Rat | Zebrafish | Fruitfly |
| GeneView | RAB38 | Rab38/R | 27987 | CG8024/Rab-RP1 |
| Protein | NP_071732 (211aa) | NP_665717 (211aa) | 04204 (167aa) | Q86PD0 (222aa) |
| Identities | 97% /205aa | 98% /208aa | 73% /114aa | 72% /127aa |
View multiple sequence alignment (PDF file) by ClustalW and GeneDoc.
Domain
(1) Domains predicted by SMART:
a) RAB: 10 - 180
(2) Transmembrane domains predicted by SOSUI: none.
(3) Graphic view of InterPro domain structure.
Motif/Site
(1) Predicted results by ScanProsite:
a) Protein kinases ATP-binding region signature :
18 - 49: LGVGKTSIIKrYvhqnfsshyratigvd..FALK
b) ATP/GTP-binding site motif A (P-loop) : [occurs frequently]
16 - 23: GdlgvGKT
c) N-myristoylation site : [occurs frequently]
19 - 24: GVgkTS,
43 - 48: GVdfAL.
d) N-glycosylation site : [occurs frequently]
33 - 36: NFSS,
74 - 77: NMTR.
e) Protein kinase C phosphorylation site : [occurs frequently]
159 - 161: SaK,
199 - 201: SpK.
f) Casein kinase II phosphorylation site : [occurs frequently]
159 - 162: SakE.
(2) Predicted results of subprograms by PSORT II:
a) N-terminal signal peptide: none
b) KDEL ER retention motif in the C-terminus: none
c) ER Membrane Retention Signals: found KKXX- like motif in the C-terminus: GCAK
d) VAC possible vacuolar targeting motif: found TLPN at 113
e) Actinin-type actin-binding motif: type 1: none; type 2: none
f) Prenylation motif: S-farnesyl cysteine at 208
g) memYQRL transport motif from cell surface to Golgi: none
h) Tyrosines in the tail: none
i) Dileucine motif in the tail: none
3D Model
(1) ModBase matched entries found, results here.
(2)ModBase predicted comparative 3D structure of Q8QZZ8 from UCSC Genome Sorter.
From left to right: Front, Top, and Side views of predicted protein.
2D-PAGE
This protein does not exist in the current release of SWISS-2DPAGE.
Computed theoretical MW=23,776Da, pI=7.65 (NP_082514).
FUNCTION
Ontology
(1) Biological process: intracellular protein transport
(2) GTPase activity.
(3) ATP binding; GTP binding
Location
Immunofluorescence staining demonstrated that expressed GST-tagged Rab38 was mainly co-localized with endoplasmic reticulum-resident protein and also partly with intermittent vesicles between the endoplasmic reticulum and the Golgi complex (Osanai, et al (2005)). Rab38 localizes to perinuclear vesicles carrying tyrosinase and tyrosinase-related protein 1 (Wasmeier, et al).
Interaction
The RAB family of proteins is comprised of small GTP-binding proteins that transition between the cytoplasm and organelle membranes and are believed to regulate vesicle transport. The two-cysteine carboxy terminal motif important for the binding of lipid moieties differs from the consensus sequence found in other RAB proteins and is more like that found in RAS proteins. Varp (VPS9-ankyrin-repeat protein)/Ankrd27 specifically binds two small GTPases, Rab32 and Rab38. A conserved Val residue in the switch II region of Rab32(Val-92) and Rab38(Val-78) is required for Varp binding activity (Tamura, et al (2009); Tamura, et al (2011)).
Rab38 drosophila homolog CG8024/Rab-RP1 interaction information in CuraGen interaction database.
Pathway
RAB38 is important in the vesicular trafficking that moves TYRP1 from the trans-Golgi network to the end-stage melanosome (Luftus, et al). Rab38 and Rab32 regulate a critical step in the trafficking of melanogenic enzymes, in particular, tyrosinase and TYRP1, from the TGN to melanosomes (Wasmeier, et al).
MUTATION
Allele or SNP
1 phenotypic allele is described in MGI:1919683.
SNPs deposited in dbSNP.
Distribution
| Location | Genomic | cDNA | Protein | Type | Strain | Reference |
| Exon 1 | 56G>T | 56G>T | G19V | missense | cht (B6) | Luftus, et al |
Effect
The G19V mutation is localized within highly conserved phosphate/Mg2+ domain and is predicted to contact GTP directly in the nucleotide binding pocket (Luftus, et al). Rab38(G19V) is inactive and that the nearly normal pigmentation in cht melanocytes results from functional compensation by the closely related Rab32 (Wasmeier, et al).
PHENOTYPE
Mutation in the Rab38 gene is the cause of recessive chocolate (cht) mutant (Luftus, et al). The cht allele arose spontaneously in the C57BL/6 strain. The strain is described in more detail in JAX Mice database (C57BL/6J-Rab38cht/J). Homozygotes have lighter eyes and skin at birth and brown rather than black hair pigment (nonagouti chocolate (a/a-cht/cht) mice). The end-stage melanosomes of chocolate mice contain less TYRP1 than do wild type mice. The cht/cht melanosomes are similar in morphology to Tyrp1b melanosomes (Luftus, et al). Mutation of both Rab38 and Tyrp1 produced mice with ocular and coat color pigment dilution greater than that seen with either mutation alone (Brooks, et al). Loss of functional Rab38 in the cht mouse causes dramatically reduced numbers of melanosomes in adult RPE, but less severe in skin melanocytes and choroidal melanocytes (Lopes, et al). The chocolate mice do not have extended bleeding times (Davisson, et al) and platelet storage defects (Luftus, et al). Cht lungs exhibited a uniform enlargement of the distal airspaces with mild alveolar destruction as well as a slight increase in lung compliance. Alveolar type II cells were engorged with lamellar bodies of increased size and number. Hydrophobic surfactant constituents (ie, phosphatidylcholine and surfactant protein B) were increased in lung tissues but decreased in alveolar spaces, consistent with a malfunction in lamellar body secretion and the subsequent cellular accumulation of these organelles (Osanai, et al (2008)).
In the rat, two HPS models are known, Fawn-hooded (FH) and Tester Moriyama (TM), non-complementing strains in which HPS-like hypopigmentation and platelet storage pool deficiency result from a mutation of the Ruby (red eyed dilution; R) locus on Chromosome (Chr) 1. FH and TM rats have identical Rab38 M1I mutations (protein-null), occurring on an identical Chr 1 marker allele haplotype, indicating that these two strains derive from a common ancestor. This ancestor appears to have been a sub-strain of the outbred Long Evans (LE) strain, and several modern LE sub-strains carry the Rab38 M1I R mutation on the same Chr 1 marker haplotype (Oiso, et al). Unlike rhe cht mice, FH rat platelets and megakaryocytes lacked dense granules (Ninkovic, et al). In LE rat HPS model, Rab38-deficient type II cells appear to carry abnormality in lung surfactant secretion but not in synthesis or uptake (Osanai, et al (2010)). A Rab38-KO rat was generated by zinc-finger nuclease (ZFN) targeting (Geurts, et al ) .
REFERENCE
- Brooks BP, Larson DM, Chan CC, Kjellstrom S, Smith RS, Crawford MA, Lamoreux L, Huizing M, Hess R, Jiao X, Hejtmancik JF, Maminishkis A, John SW, Bush R, Pavan WJ. Analysis of ocular hypopigmentation in Rab38cht/cht mice.Invest Ophthalmol Vis Sci 2007; 48:3905-13. PMID: 17724166
- Davisson MT, Bunker HP. Linkage of Chocolate (cht) on Chr 7. Mouse News Lett 1986; 75: 31. (No Abstract)
- Geurts AM, Cost GJ, Freyvert Y, Zeitler B, Miller JC, Choi VM, Jenkins SS, Wood A, Cui X, Meng X, Vincent A, Lam S, Michalkiewicz M, Schilling R, Foeckler J, Kalloway S, Weiler H, M¨Śnoret S, Anegon I, Davis GD, Zhang L, Rebar EJ, Gregory PD, Urnov FD, Jacob HJ, Buelow R. Knockout rats via embryo microinjection of zinc-finger nucleases. Science 2009; 325:433. PMID: 19628861
- Loftus SK, Larson DM, Baxter LL, Antonellis A, Chen Y, Wu X, Jiang Y, Bittner M, Hammer JA 3rd, Pavan WJ. Mutation of melanosome protein RAB38 in chocolate mice. Proc Natl Acad Sci U S A 2002; 99: 4471-6. PMID: 11917121
- Lopes VS, Wasmeier C, Seabra MC, Futter CE. Melanosome maturation defect in Rab38-deficient retinal pigment epithelium results in instability of immature melanosomes during transient melanogenesis. Mol Biol Cell 2007; 18: 3914-27. PMID: 17671165
- Ninkovic I, White JG, Rangel-Filho A, Datta YH. The role of Rab38 in platelet dense granule defects. J Thromb Haemost 2008; 6: 2143-51. PMID: 18983523
- Oiso N, Riddle SR, Serikawa T, Kuramoto T, Spritz RA. The rat Ruby ( R) locus is Rab38: identical mutations in Fawn-hooded and Tester-Moriyama rats derived from an ancestral Long Evans rat sub-strain. Mamm Genome 2004; 15: 307-14. PMID: 15112108
- Osanai K, Takahashi K, Nakamura K, Takahashi M, Ishigaki M, Sakuma T, Toga H, Suzuki T, Voelker DR. Expression and characterization of Rab38, a new member of the Rab small G protein family. Biol Chem 2005; 386: 143-53. PMID: 15843158
- Osanai K, Oikawa R, Higuchi J, Kobayashi M, Tsuchihara K, Iguchi M, Jongsu H, Toga H, Voelker DR. A mutation in Rab38 small GTPase causes abnormal lung surfactant homeostasis and aberrant alveolar structure in mice. Am J Pathol 2008; 173: 1265-74. PMID: 18832574
- Osanai K, Higuchi J, Oikawa R, Kobayashi M, Tsuchihara K, Iguchi M, Huang J, Voelker DR, Toga H. Altered lung surfactant system in a Rab38-deficient rat model of Hermansky-Pudlak syndrome. Am J Physiol Lung Cell Mol Physiol 2010; 298: L243-51. PMID: 19897744
- Tamura K, Ohbayashi N, Maruta Y, Kanno E, Itoh T, Fukuda M. Varp is a novel Rab32/38-binding protein that regulates Tyrp1 trafficking in melanocytes. Mol Biol Cell 2009; 20: 2900-8. PMID: 19403694
- Tamura K, Ohbayashi N, Ishibashi K, Fukuda M. Structure-function analysis of VPS9-ankyrin-repeat protein (Varp) in the trafficking of tyrosinase-related protein 1 in melanocytes. J Biol Chem 2011; 286: 7507-21. PMID: 21187289
- Wasmeier C, Romao M, Plowright L, Bennett DC, Raposo G, Seabra MC. Rab38 and Rab32 control post-Golgi trafficking of melanogenic enzymes. J Cell Biol. 2006; 175: 271-81. PMID: 17043139
EDIT HISTORY:
Created by Wei Li and Johnathan Bourne, 07/23/2004
Updated by Wei Li, 12/28/2006
Updated by Wei Li, 02/22/2008
Updated by Wei Li, 03/12/2009
Updated by Wei Li, 05/29/2011