GENOMIC
Mapping
7qD3. View the map and BAC clones (data from UCSC genome browser).
Structure
(assembly 10/03)
Tyr/NM_011661: 5 exons, 66,007bp, Chr7: 79,815,461 - 79,881,467.
The figure below shows the structure of the Tyr gene (data from UCSC genome browser).
Regulatory Element
Search the 5'UTR and 1kb upstream regions (human and mouse) by CONREAL with 80% Position Weight Matrices (PWMs) threshold (view results here).
TRANSCRIPT
RefSeq/ORF
Tyr (NM_011661), 3,308bp, view ORF and the alignment to genomic.
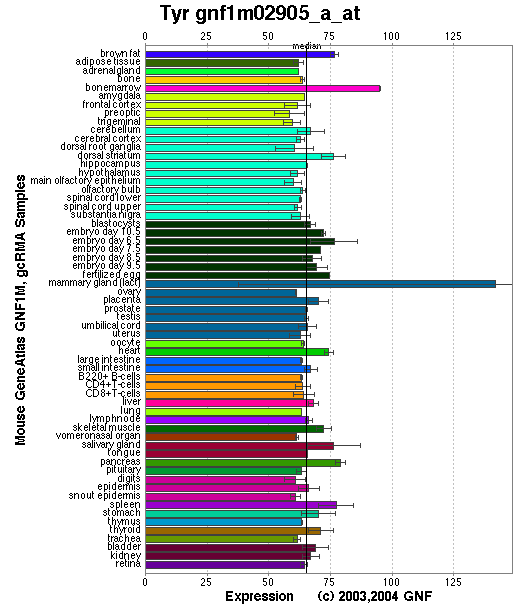
Expression Pattern
Tissue specificity: highly expressed in pigment cells.
Affymetrix microarray expression pattern in SymAtlas from GNF is shown below.
PROTEIN
Sequence
Tyrosinase [precursor] (NP_035791): 533aa, ExPaSy NiceProt view of Swiss-Prot:P11344.
Synonym: Monophenol monooxygenase; Albino locus protein.
Enzyme: EC 1.14.18.1.
Ortholog
| Species | Human | Cow | Rat |
| GeneView | OCA1/TYR | TYR | Tyr |
| Protein | NP_000363 (529aa) | NP_851344(530aa) | XP_238901(530aa) |
| Identities | 85% /457aa | 81% /435aa | 95% /508aa |
| Species | Chicken | Zebrafish | Worm |
| GeneView | TYR | tyr | C34G6.2 |
| Protein | NP_989491(529aa) | NP_571088(535aa) | WP:CE08574(751aa) |
| Identities | 71% /382aa | 61% /325aa | 30% /80aa |
View multiple sequence alignment (PDF file) by ClustalW and GeneDoc.
Domain
(1) Domains predicted by SMART:
a) signal peptide 1-15
b) Pfam: Tyrosinase 111-456
c) tansmembrane 474-498
(2) Transmembrane domains predicted by SOSUI: Two transmembrane helices detected.
| No. | N terminal | transmembrane region | C terminal | type | length |
| 1 | 1 | MFLAVLYCLLWSFQISDGHFPR | 22 | SECONDARY | 22 |
| 2 | 476 | PWLLGAALVGAVIAAALSGLSSR | 498 | PRIMARY | 23 |
(3) Graphical view of InterPro domain structure.
Motif/Site
(1) Predicted results by ScanProsite:
a) EGF-like domain signature 1 :
89 - 100: CqCsgnfmGfnC
b) Laminin-type EGF-like (LE) domain signature :
89 - 112: Cq.CsgnfmGfnCgnCkfg.Fggpn...........C
c) Tyrosinase CuA-binding region signature :
202 - 219: Heapg.FLpWHRlfLllwE
d) Tyrosinase and hemocyanins CuB-binding region signature :
383 - 394: DPiFLlhHafvD
e) Protein kinase C phosphorylation site : [occurs frequently]
26 - 28: SsK,
50 - 52: SgR,
113 - 115: TeK,
129 - 131: SeK,
309 - 311: TpR,
339 - 341: SfR,
441 - 443: TsK,
496 - 498: SsR.
f) N-myristoylation site : [occurs frequently]
43 - 48: GSpcGQ,
47 - 52: GQlsGR,
93 - 98: GNfmGF,
108 - 113: GGpnCT,
157 - 162: GQmnNG,
485 - 490: GAviAA.
g) N-glycosylation site : [occurs frequently]
86 - 89: NRTC,
111 - 114: NCTE,
161 - 164: NGST,
230 - 233: NFTV,
337 - 340: NFSF,
371 - 374: NGTM.
h) Microbodies C-terminal targeting signal : [occurs frequently]
531 - 533: SHL
i) Tyrosine sulfation site : [occurs frequently]
442 - 456:
skdlgydYsylqesd
(2) Predicted results of subprograms by PSORT II:
a) Seems to have a cleavable signal peptide (1 to 14)
b) KDEL ER retention motif in the C-terminus: none
c) ER Membrane Retention Signals: none
d) VAC possible vacuolar targeting motif: none
e) Actinin-type actin-binding motif: type 1: none; type 2: none
f) Prenylation motif: none
g) memYQRL transport motif from cell surface to Golgi: none
h) Tyrosines in the tail: found at 523 and 528
i) Dileucine motif in the tail: found LL at 517 and 527
3D Model
(1) ModBase entry found, results here.
(2)ModBase Predicted Comparative 3D Structure of P11344 from UCSC Genome Sorter.
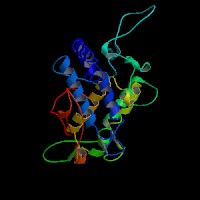
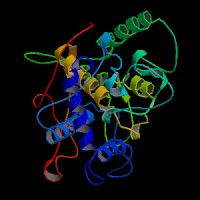
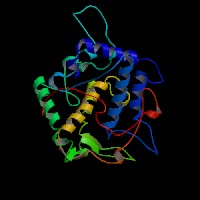
From left to right: Front, Top, and Side views of predicted protein
2D-PAGE
This protein does not exist in the current release of SWISS-2DPAGE.
Computed theoretical MW=60,606Da, pI=5.67 (NP_035791, precursor).
FUNCTION
Ontology
a) Biological process: melanin biosynthesis from tyrosine
b) Monophenol monooxygenase activity
c) Component of integral to membrane
Location
Integral membrane protein. Melanosome.
Interaction
Tyrosinase binds 2 copper ions per subunit (CuA and CuB). Tyrosinase is activated when protein kinase C-beta (PKC-beta) phosphorylates the serine residues at amino acid positions 505 and 509. Phosphorylation of tyrosinase by PKC-beta induces a complex formation between tyrosinase and TYRP1 (Wu, et al). The p protein regulates posttranslational processing of tyrosinase, and hypopigmentation in melan-p1 cells is the result of altered tyrosinase processing and trafficking (Chen, et al; Toyofuku, et al).
Pathway
Tyrosinase (Monophenol monooxygenase, EC 1.14.18.1) is a glycoprotein and a copper-containing oxidase that functions in the formation of pigments such as melanins and other polyphenolic compounds. It catalyzes the rate-limiting conversions of tyrosine to dopa, dopa to dopa-quinone and possibly 5,6-dihydroxyindole to indole-5,6 quinone (L-tyrosine + L-DOPA + O2 = L-DOPA + DOPAquinone + H2O).
During the melanogenesis, tyrosinase can be released from the ER in the presence of protonophore or proton pump inhibitors which increase the pH of intracellular organelles, after which tyrosinase is transported correctly to the Golgi, and then to melanosomes via the endosomal sorting system (Watabe, et al). Tyrosinase is sorted via the dilucine signal to the melanosomes for melanin synthesis mediated by AP-3 complex (view diagram of melanosome maturation in melanocytes here).
KEGG Biochemical Pathways:
(1) mmu00350 - Tyrosine metabolism - Mus musculus
(2) mmu00940 - Flavonoids, stilbene and lignin biosynthesis - Mus musculus
MUTATION
Allele or SNP
103 phenotypic alleles described in MGI:98880.
Distribution
Among the 25 spontaneous mutations, the allelic c, c-2J, c-1R, c-Bc, c-Bc2, c-Bc3, c-ch, c-h, and c-p have been characterized molecularly (details in MGI:98880).
Effect
The identified 9 spontaneous mutations so far are missense mutations or gross deletions/insertions to disrupt the function of tyrosinase.
PHENOTYPE
Defects in Tyr are the cause of albino (c) mutants, an autosomal recessive disorder similar to OCA1 (OMIM 203100). Numerous spontaneous and induced mutations at this locus result in albinism or hypopigmentation due to absence or reduction of melanin in homozygotes. Albinism is associated with reduced number of optic nerve fibers and mutants can have impaired vision. For more details of the albino phenotype, view the Mouse Locus Catalog #Tyr. The original c mutation arose spontaneously. This very old mutant was already known in Greek and Roman times. The strain is described in more detail in JAX Mice database (B6.Cg-Tyrc-J/J).
A missense substitution at codon R299H in exon 2 of the Tyr gene causes the albino Wistar rat (Blaszczyk, et al).
REFERENCE
- Blaszczyk WM, Arning L, Hoffmann KP, Epplen JT. A Tyrosinase missense mutation causes albinism in the Wistar rat. Pigment Cell Res 2005; 18: 144-5. PMID: 15760344
- Chen K, Manga P, Orlow SJ. Pink-eyed dilution protein controls the processing of tyrosinase. Mol Biol Cell 2002; 13: 1953-64. PMID: 12058062
- Toyofuku K, Valencia JC, Kushimoto T, Costin GE, Virador VM, Vieira WD, Ferrans VJ, Hearing VJ. The etiology of oculocutaneous albinism (OCA) type II: the pink protein modulates the processing and transport of tyrosinase. Pigment Cell Res 2002; 15: 217-24. PMID: 12028586
- Watabe H, Valencia JC, Yasumoto K, Kushimoto T, Ando H, Muller J, Vieira WD, Mizoguchi M, Appella E, Hearing VJ. Regulation of tyrosinase processing and trafficking by organellar pH and by proteasome activity. J Biol Chem 2004; 279: 7971-81. PMID: 14634018
- Wu H, Park HY. Protein kinase C-beta-mediated complex formation between tyrosinase and TRP-1. Biochem Biophys Res Commun 2003; 311: 948-53. PMID: 14623273
EDIT HISTORY:
Created by Wei Li & Jonathan Bourne: 07/26/2004
Updated by Wei Li: 04/06/2006